If the maximum kinetic energy of emitted photo electrons from a metal surface of work function 2.5 eV, is 1.7 eV. If wavelength of incident radiation is halved, then stopping potential will be
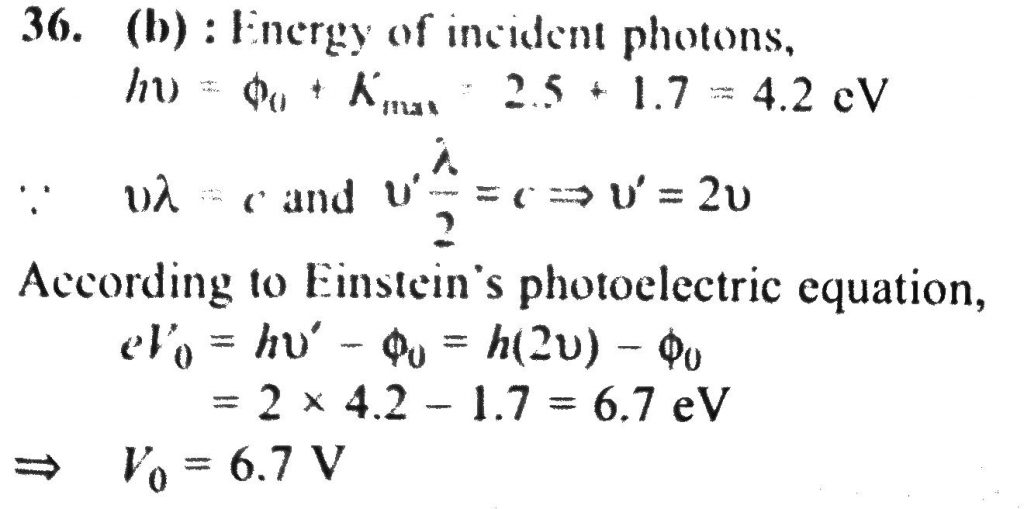
- November 28, 2020
- Category: AIIMS Last 24 Years Solved 1996 - 2019 Physics and Chemistry Video Solutions , Chapter 29 - Dual Nature of Matter and Radiation , Physics ,
Related
If Kinetic energy of the emitted electrons is zero when light of wavelength 686 nm. Then wavelength of radiations emitted by electron having energy 1.81 eV will be. ( Given work function of Cesium = 1.81 eV)
The kinetic energy of most energetic electrons emitted from a metallic surface is doubled when the wavelength λ of the incident radiation is changed from 400 nm to 310 nm. The work function of the metal is
Radiation of wavelength 546 nm falls on a photo cathode and electrons with maximum kinetic energy of 0.18 eV are emitted. When radiation of wavelength 185 nm falls on the same surface,
[vc_row][vc_column][stm_post_info css=".vc_custom_1437111129257{margin-bottom: 0px !important;}"][vc_row_inner][vc_column_inner width="1/6"][/vc_column_inner][vc_column_inner width="1/6"][/vc_column_inner][/vc_row_inner][vc_row_inner][vc_column_inner width="1/6"][/vc_column_inner][vc_column_inner width="2/3"][vc_single_image image="58279" img_size="large"][vc_single_image image="58284" img_size="large"][vc_single_image image="58288" img_size="large"][/vc_column_inner][vc_column_inner width="1/6"][/vc_column_inner][/vc_row_inner][vc_row_inner][vc_column_inner width="1/2"][stm_post_tags][/vc_column_inner][vc_column_inner width="1/2"][stm_share code="JTNDc3BhbiUyMGNsYXNzJTNEJTI3c3RfZmFjZWJvb2tfbGFyZ2UlMjclMjBkaXNwbGF5VGV4dCUzRCUyNyUyNyUzRSUzQyUyRnNwYW4lM0UlMEElM0NzcGFuJTIwY2xhc3MlM0QlMjdzdF90d2l0dGVyX2xhcmdlJTI3JTIwZGlzcGxheVRleHQlM0QlMjclMjclM0UlM0MlMkZzcGFuJTNFJTBBJTNDc3BhbiUyMGNsYXNzJTNEJTI3c3RfZ29vZ2xlcGx1c19sYXJnZSUyNyUyMGRpc3BsYXlUZXh0JTNEJTI3JTI3JTNFJTNDJTJGc3BhbiUzRSUwQSUzQ3NwYW4lMjBjbGFzcyUzRCUyN3N0X3NoYXJldGhpc19sYXJnZSUyNyUyMGRpc3BsYXlUZXh0JTNEJTI3JTI3JTNFJTNDJTJGc3BhbiUzRQ=="][/vc_column_inner][/vc_row_inner][stm_post_author][stm_post_comments][/vc_column][/vc_row]
