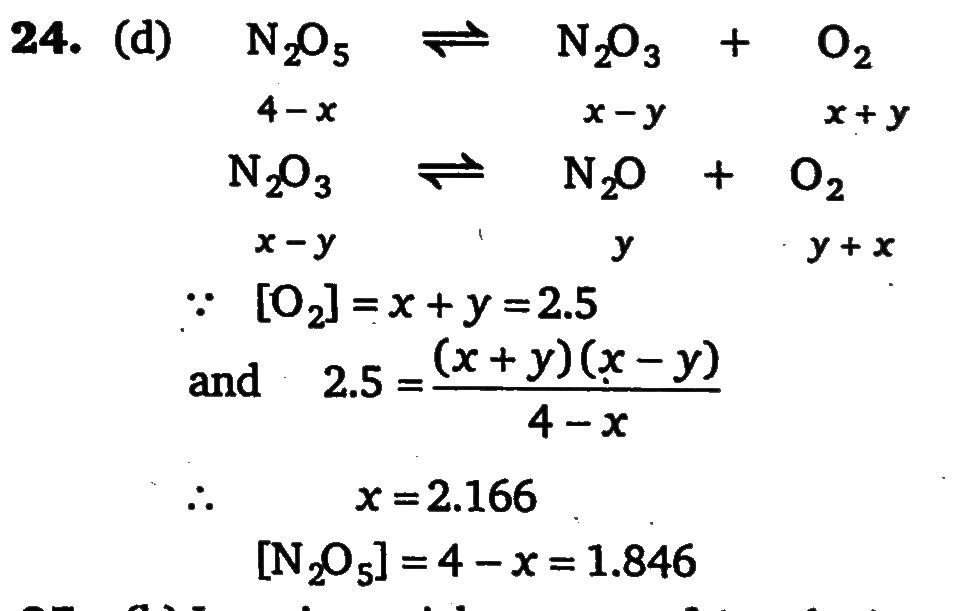When N2O5 is heated at certain temperature, it dissociates as N2O5 (g)⇌N2O3 (g)+O 2 (g);Kc =2.5. At the same time N2O3 also decomposes as: N2 O3 (g)⇌N 2O+O 2(g). If initially 4.0 moles of N2O 5 are taken in 1.0 litre flask and allowed to dissociation, concentration of O2 at equilibrium is 2.5M. Equilibrium concentration of N2O5 is: