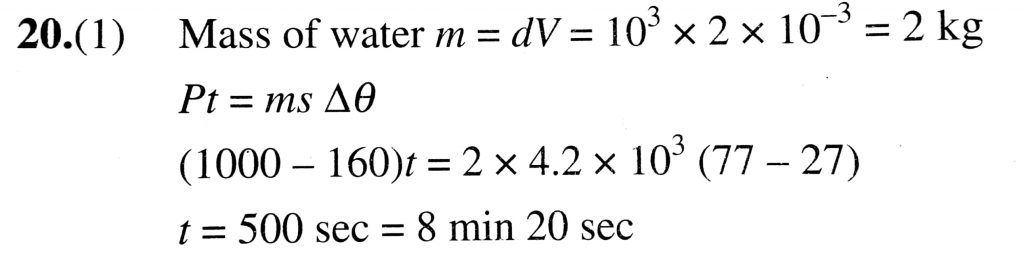Water of volume 2 litre in a container is heated with a coil of 1kW at 27^∘C. The lid of the container is open and energy dissipates at rate of 160J/s. In how much time temperature will rise from 27^∘C to 77^∘C [Given specific heat of water is 4.2kJ/kg degree C]