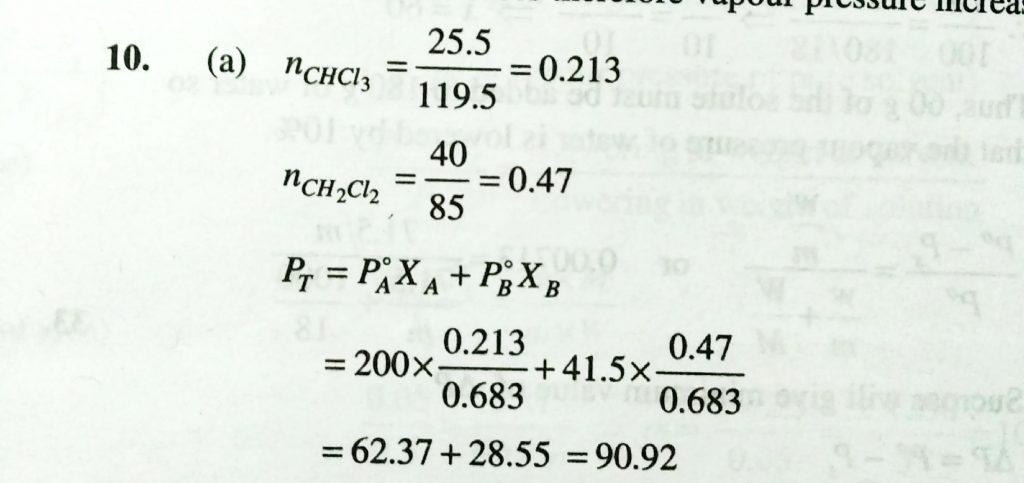Vapour pressure of chloroform ( CHCl3 ) and dichloromethane (CH2CL2 ) at 25 degree C are 200 mm Hg and 415 mm Hg respectively. Vapour pressure of the solution obtained by mixing 25.5 g of CHCl 3 and 40 g of CH 2Cl 2 at the same temperature will be: [Molecular mass of CHCl 3 = 119.5 g/mol and molecular mass of CH 2 Cl 2 =85 g/ mol ]