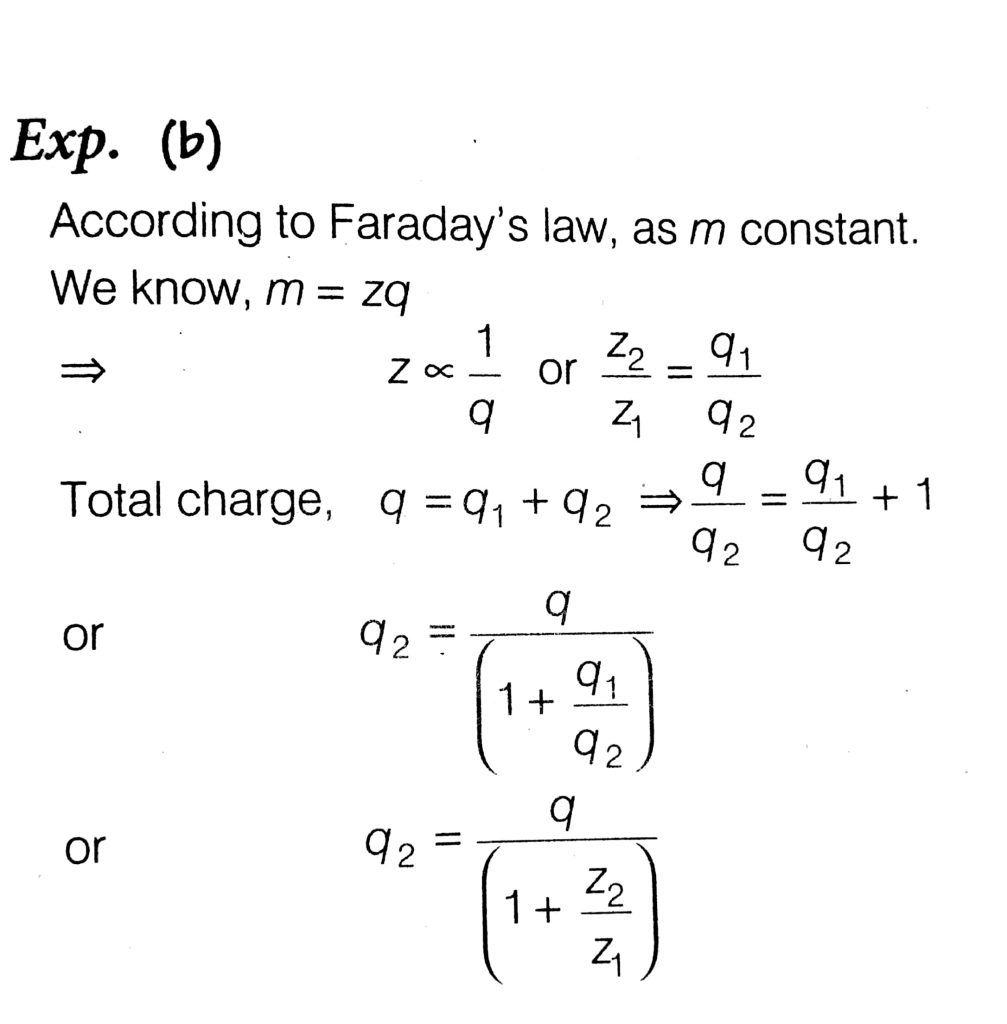
Two voltmeters, one of copper and another of silver are joined in parallel. When a total charge q flows through voltmeters, equal amount of metals are deposited. If the electrochemical equivalents of Cu and Ag are z1 and z2 respectively then charges flows through the silver voltmeter is