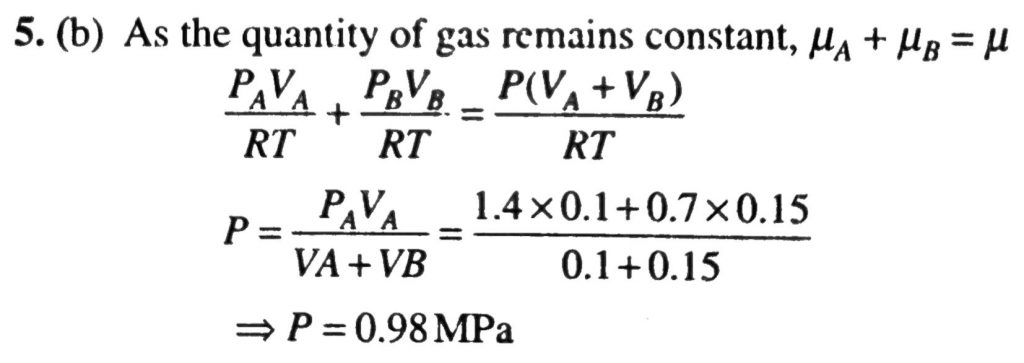Two gases occupy two containers A and B the gas in A, of volume 0.10m^3, exerts a pressure of 1.40 MPa and that in B of volume 0.15m^3 exerts a pressure 0.7 MPa. The two containers are united by a tube of negligible volume and the gases are allowed to intermingle. Then if the temperature remains constant, the final pressure in the container will be (in MPa)