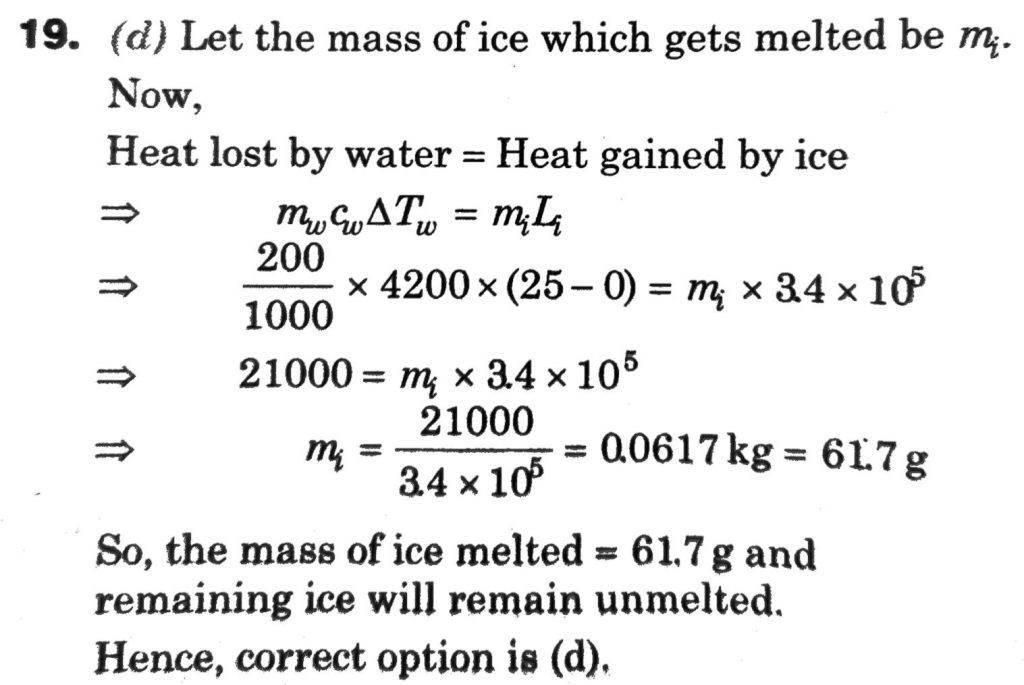The specific heat of water = 4200 J kg^–1 K^–1 and the latent heat of ice = 3.4 × 10^5 J kg^–1. 100 grams of ice at 0°C is placed in 200 g of water at 25°C. The amount of ice that will melt as the temperature of water reaches 0°C is close to (in grams)