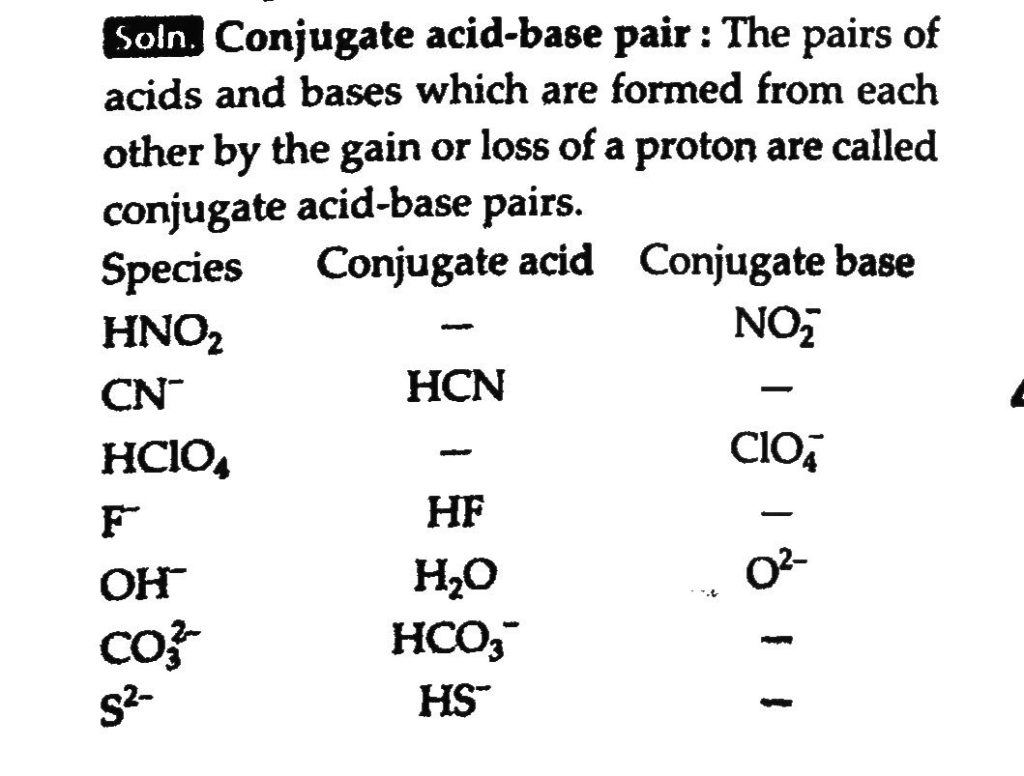
The reaction COg + 3H2g ↔ CH4g + H2O g is at equilibrium at 1300 K in a 1L flask. It also contain 0.30 mol of CO 0.10 mol of H2 and 0.02 mol of H2O and an unknown amount of CH4 in the flask. Determine the concentration of CH4 in the mixture. The equilibrium constant Kc for the reaction at the given temperature is 3.90.