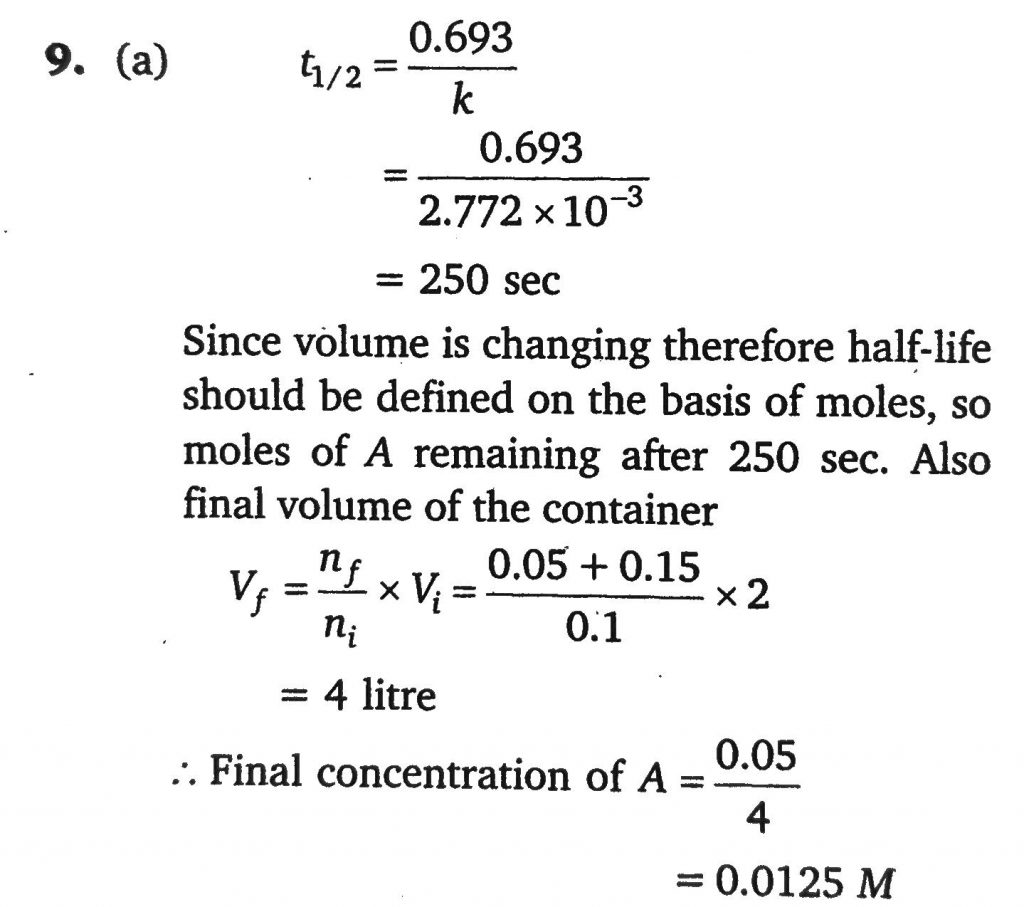
The reaction A(g)→B(g)+2C(g) is a first order reaction with rate constant 2.772×10^−3 sec^−1. Starting with 0.1 mol of A in 2 litre vessel, find the concentration of A after 250 sec when the reaction is allowed to take place at constant pressure and at 300 K ?