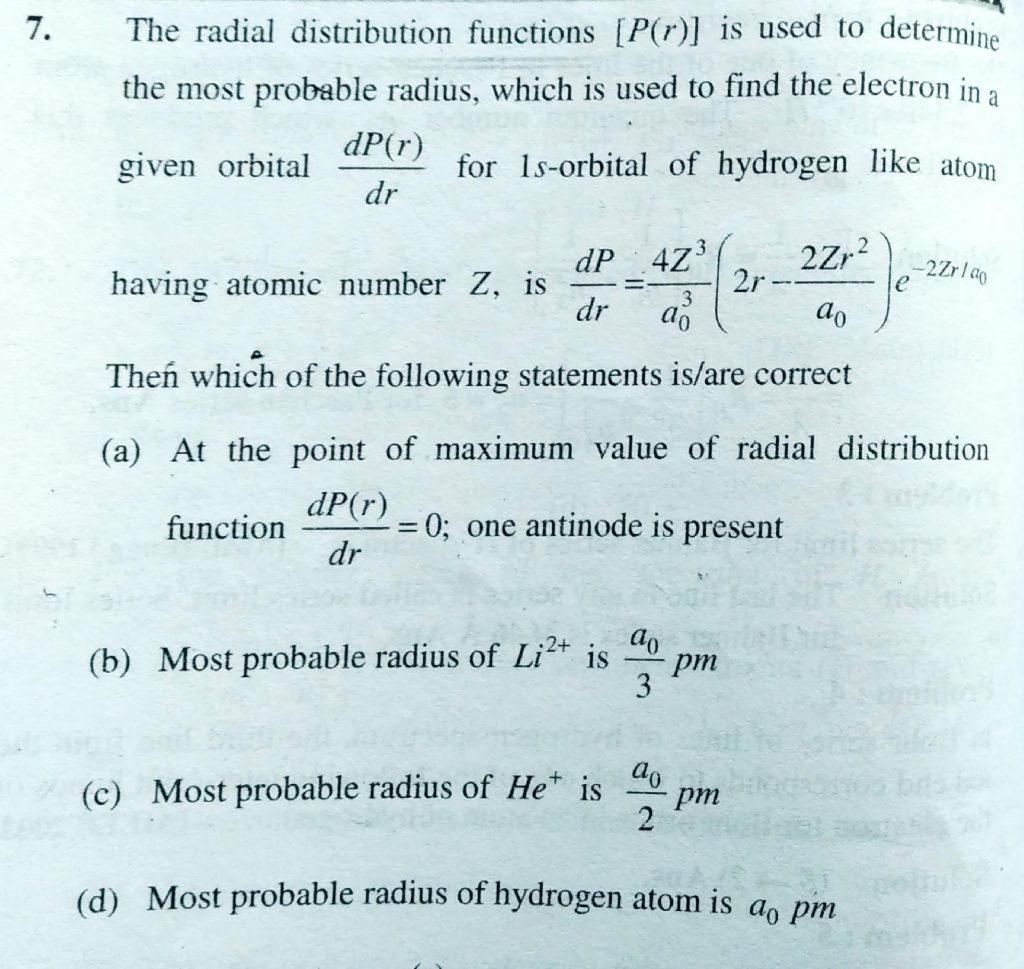
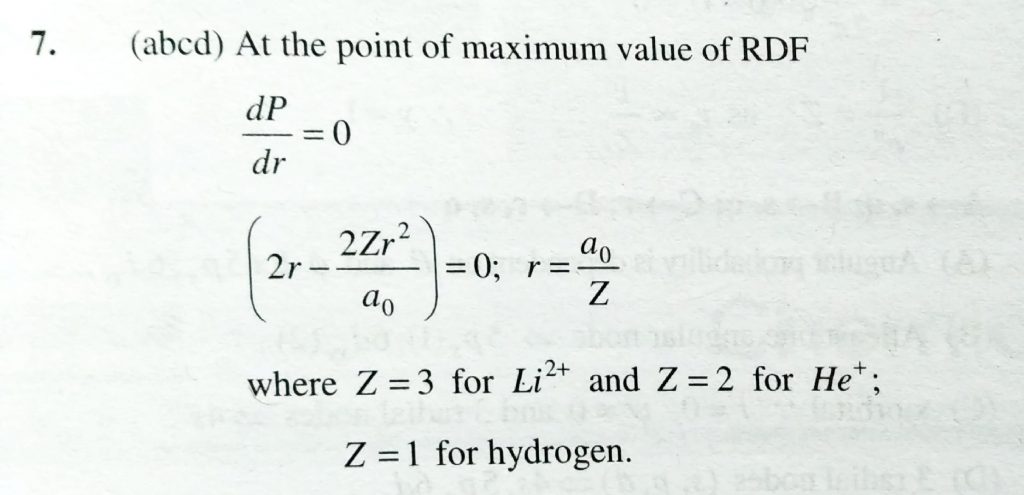The radial distribution functions [P(r)] is used to determine the most probable radius, which is used to find the electron in a given orbital dP(r)dr for 1s-orbital of hydrogen like atom having atomic number Z, is dP/dr = 4Z^3/a^30( 2 r−2Zr^2/a0 )e−2Zr/a0 :