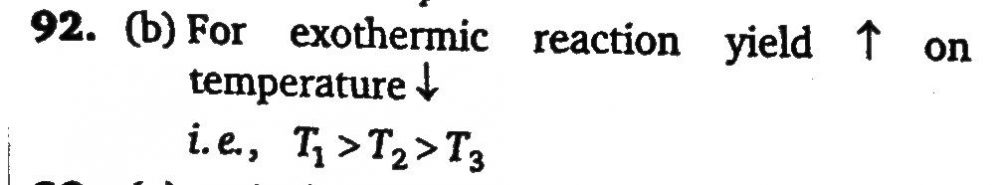The preparation of SO3 (g) by reaction SO2 (g)+ 2/1 O2 ⇌ SO3 (g) is an exothermic reaction. If the preparation follows the shown temperature-pressure relationship for its % yield at temperatures T1 ,T2 and T3, then the correct relation between T1 ,T2 and T3 is: