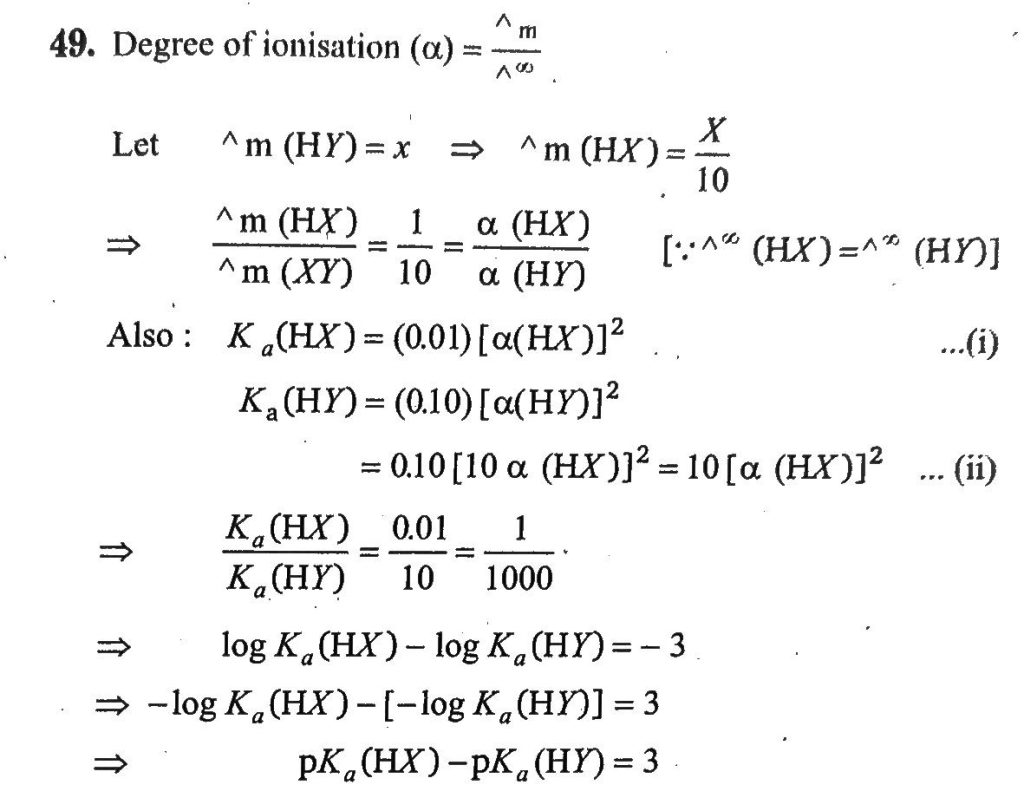
The molar conductivity of a solution of a weak acid HX(0.01M) is 10 times smaller than the molar conductivity of a solution of a weak acid HY(0.10M). If λ∘X−=λ∘Y−, the difference in their pKa values, pKa(HX)−pKa(HY), is (consider degree of ionisation of both acids to be