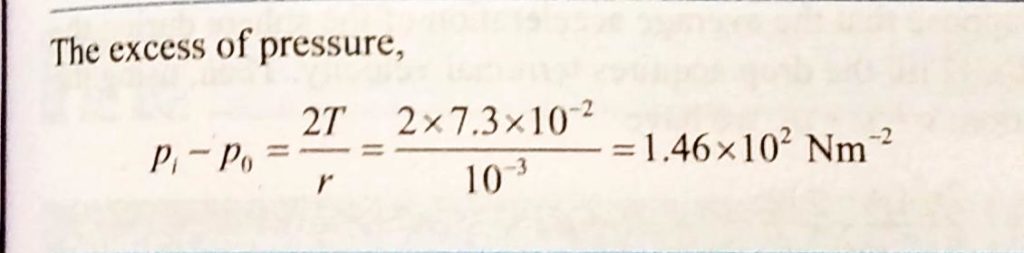The lower end of a capillary tube of diameter 2.0 mm is dipped 8.00 cm below the surface of water in a beaker. What is the pressure required in the tube in order to blow a hemispherical bubble at its end in water? The surface tension of water at temperature of the experiments is 7.30×10−2Nm−1. 1 atmospheric pressure =1.01×105 Pa, density of water =1000 kg/m^3,g=9.80 ms^−2. also calculate the excess pressure.