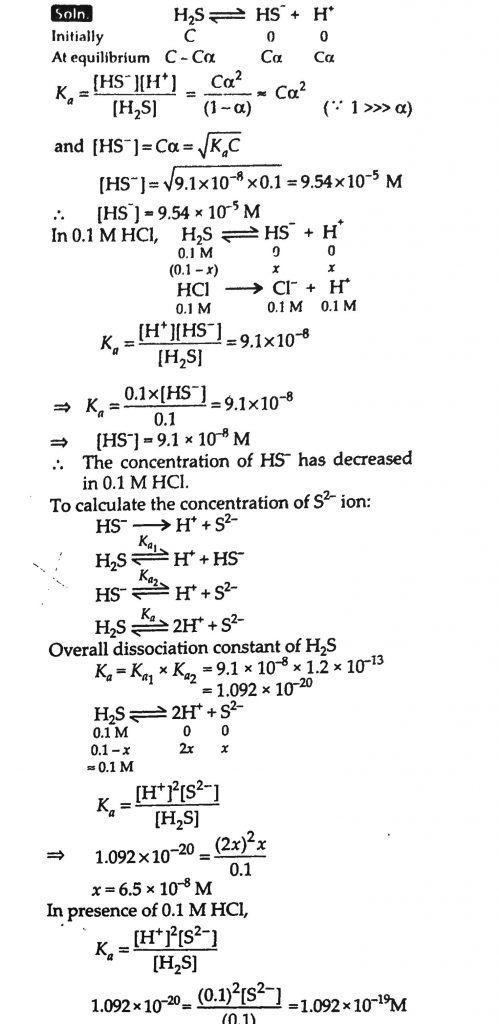
The first ionization constant of H2S is 9.1×10 −8. Calculate the concentration of HS − ion in its 0.1 M solution. How will this concentration be affected if the solution is 0.1M in HCl also? If the second dissociation constant of H 2 S is 1.2×10 −13, calculate the concentration of S.2−under both conditions.