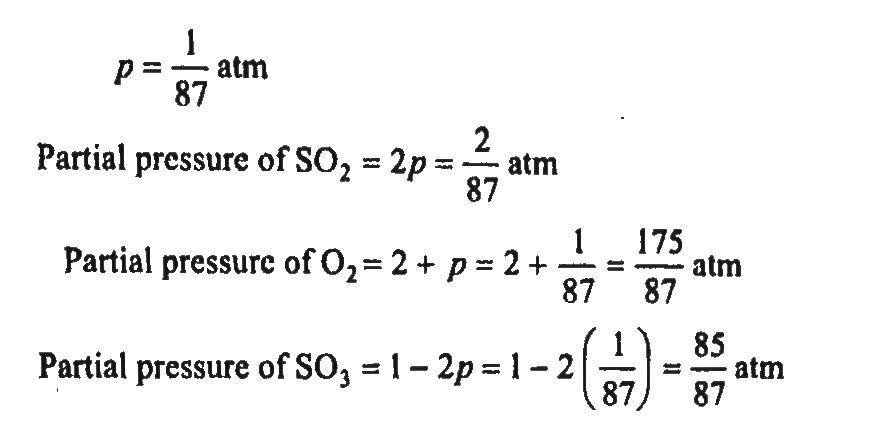
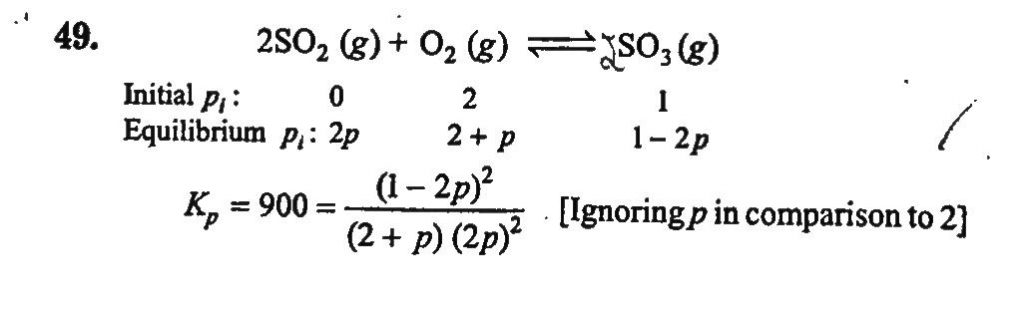The equilibrium constant Kp of the reaction, 2SO2(g) + O2(g) ⇋ 2SO3(g) is 900 atm at 800 K. A mixture containing SO3 and O2 having initial pressure of 1 and 2 atm respectively is heated at constant volume to equilibrate. Calculate the partial pressure of each gas at 800 K.