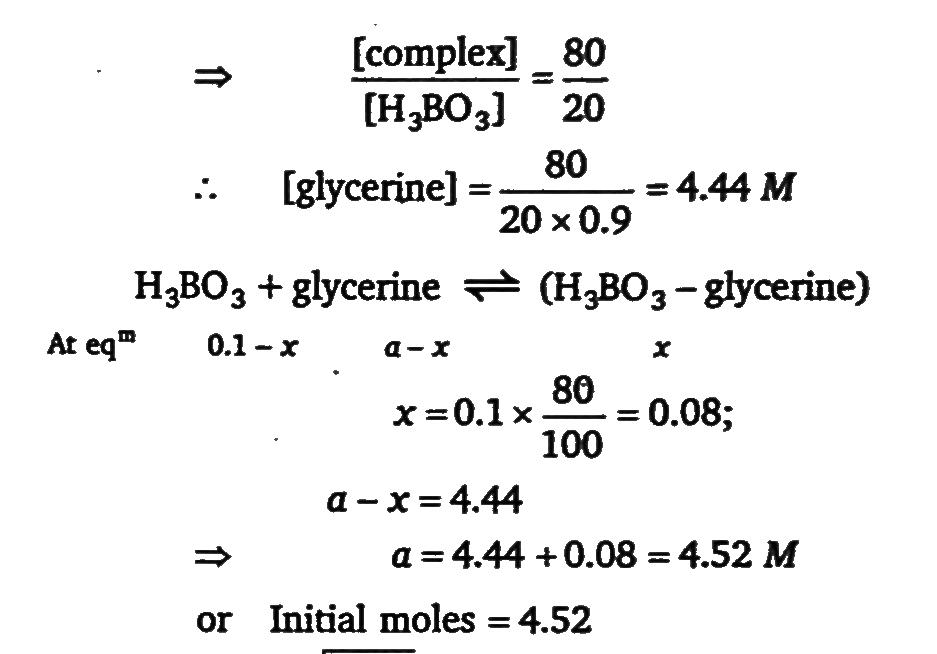
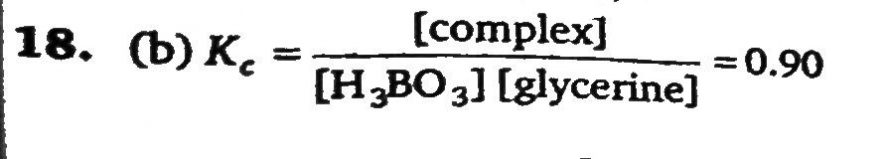The equilibrium constant for the reaction in aqueous solution H3BO3 + glycerin ⇌ H3BO3 - glycerin is 0.90. How many moles of glycerin should be added per litre of 0.10 M H3 BO3 so that 80% of the H3 BO3 is converted to the boric-acid-glycerin complex?