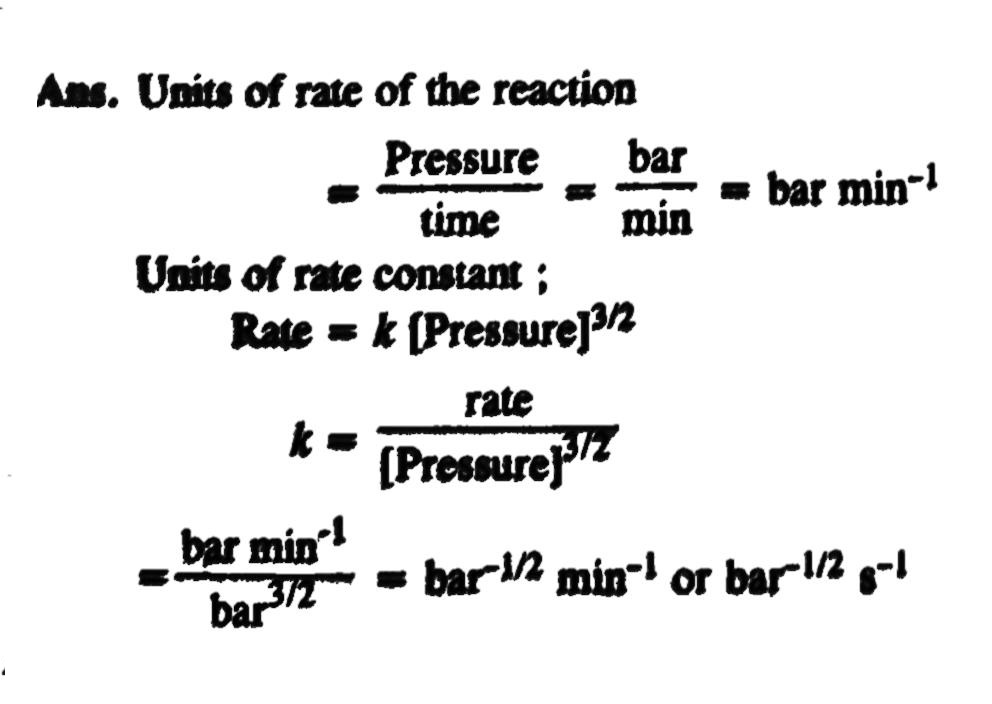
The decomposition of dimethyl ether leads to the formation of CH4, H2 and CO and the reaction rate is given by the expression: rate =k[CH3COOH3]l3/2 The rate of reaction is followed by increase in pressure in a close vessel and the rate can also be expressed in terms of partial pressure of dimethyl ether: rate =k[CH3OCH3]3/2 If the pressure is measured in bar and time in minutes, then what are the units of rate and rate constant?