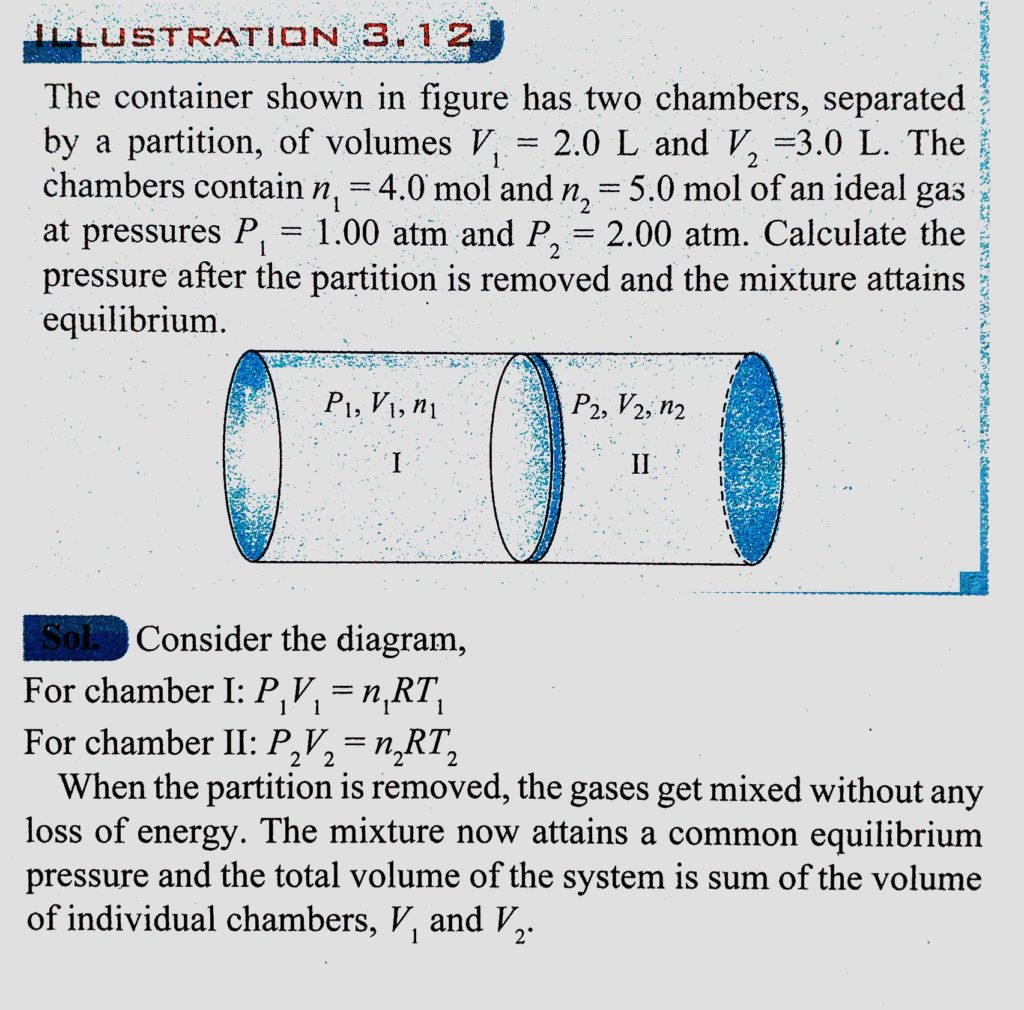
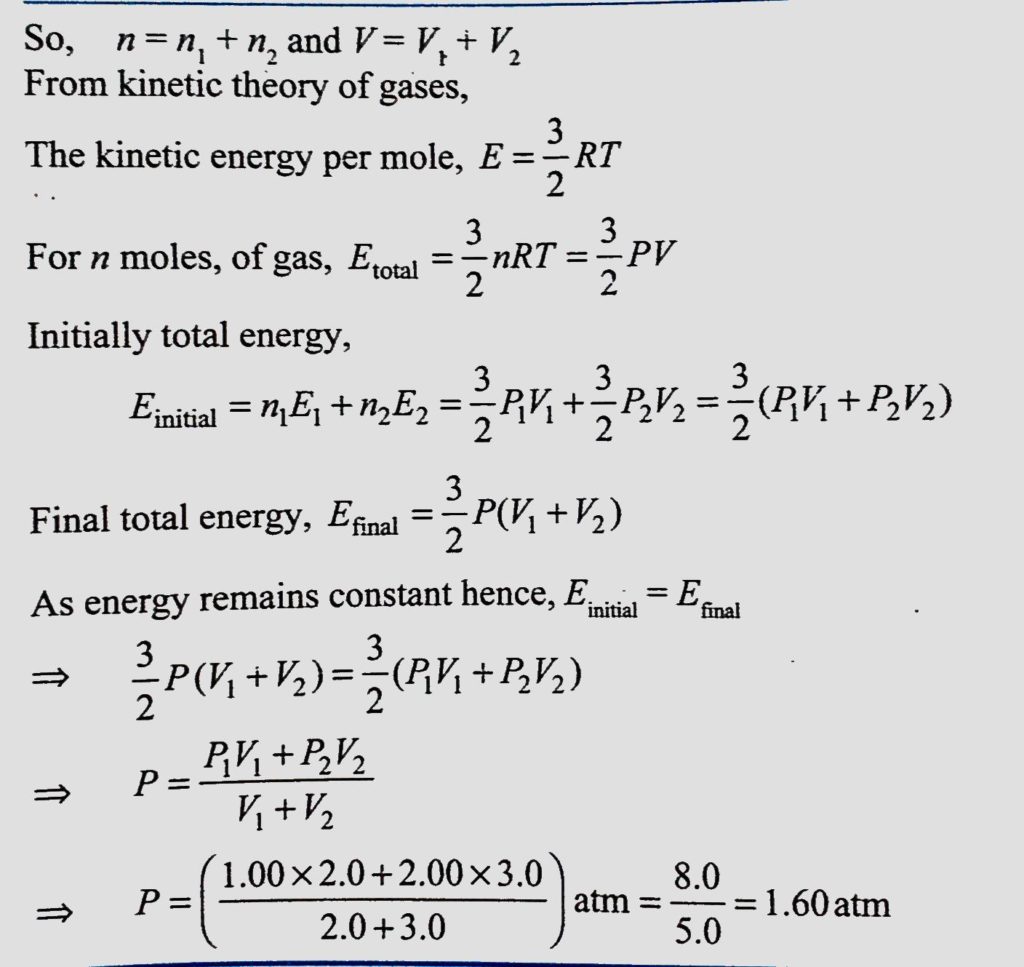
The container shown in figure has two chambers separated by a partition of volumes V1 = 2.0L and V2 = 3.0L. The chambers contain μ14.0 and μ2 = 5.0 mole of a gas at pressure p1 = 1.00 atm and P2=2.00 atm. Calculate the pressure after the partition is removed and the mixture attains equilibrium.