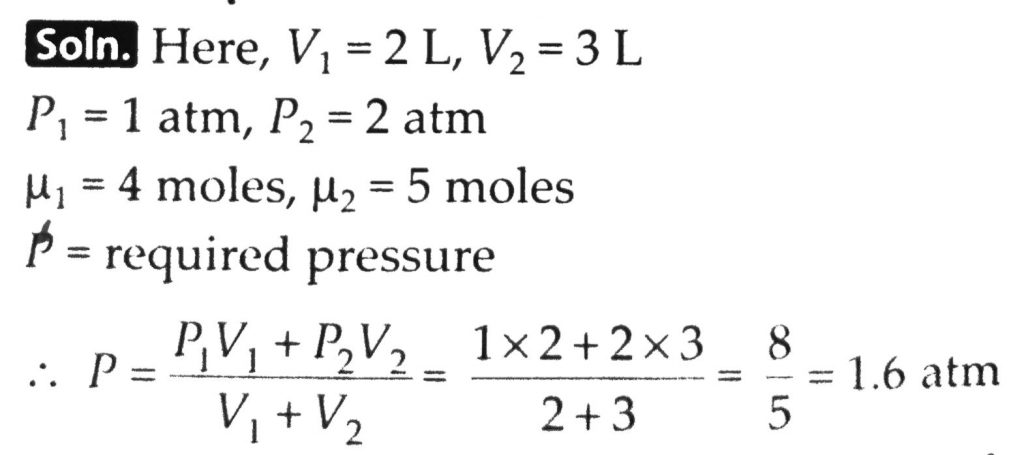The container shown in Fig. 13.6 has two chambers, separated by a partition, of volumes V1 = 2.0 litre and V2= 3.0 litre. The chambers contain μ1 = 4.0 and μ2 = 5.0 moles of a gas at pressures p1 = 1.00 atm and p2 = 2.00 atm. Calculate the pressure after the partition is removed and the mixture attains equilibrium.