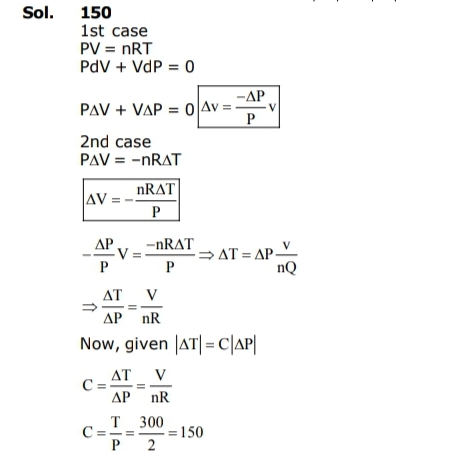
The change in the magnitude of the volume of an ideal gas when a small additional pressure ΔP is applied at a constant temperature, is the same as the change when the temperature is reduced by a small quantity ΔT at constant pressure. The initial temperature and pressure of the gas were 300 K and 2 atm. respectively. If |ΔT| = C|ΔP| then value of C in (K/atm.) is ____.