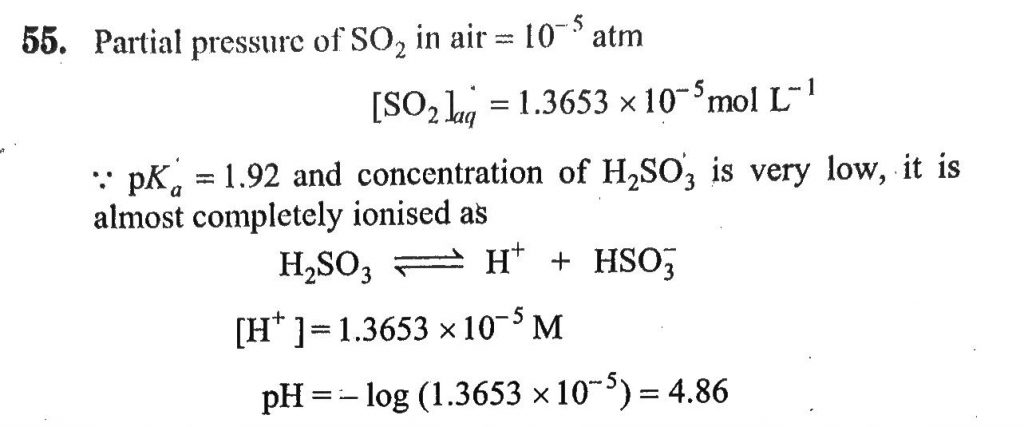
The average concentration of SO2 in the atmosphere over a city on a certain day is 10 ppm, when the average temperature is 298 K. Given that the solubility of SO2 in water at 298 K is 1.3653 mol litre^-1 and the pKa of H2SO3 is 1.92, estimate the pH of rain on that day.