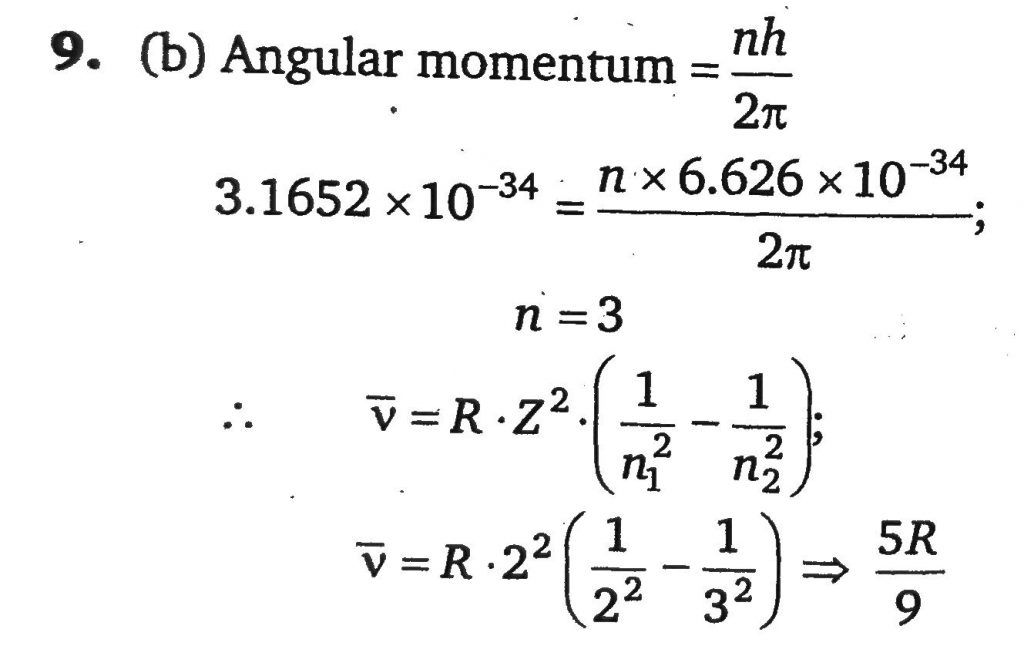The angular momentum of an electron in a Bohr’s orbit of He+ is 3.1652 × 10^−34 kg−m^2/sec. What is the wave number in terms of Rydberg constant (R) of the spectral line emitted when an electron falls this level to the first excited state. [Use h = 6.626×10^−34Js].