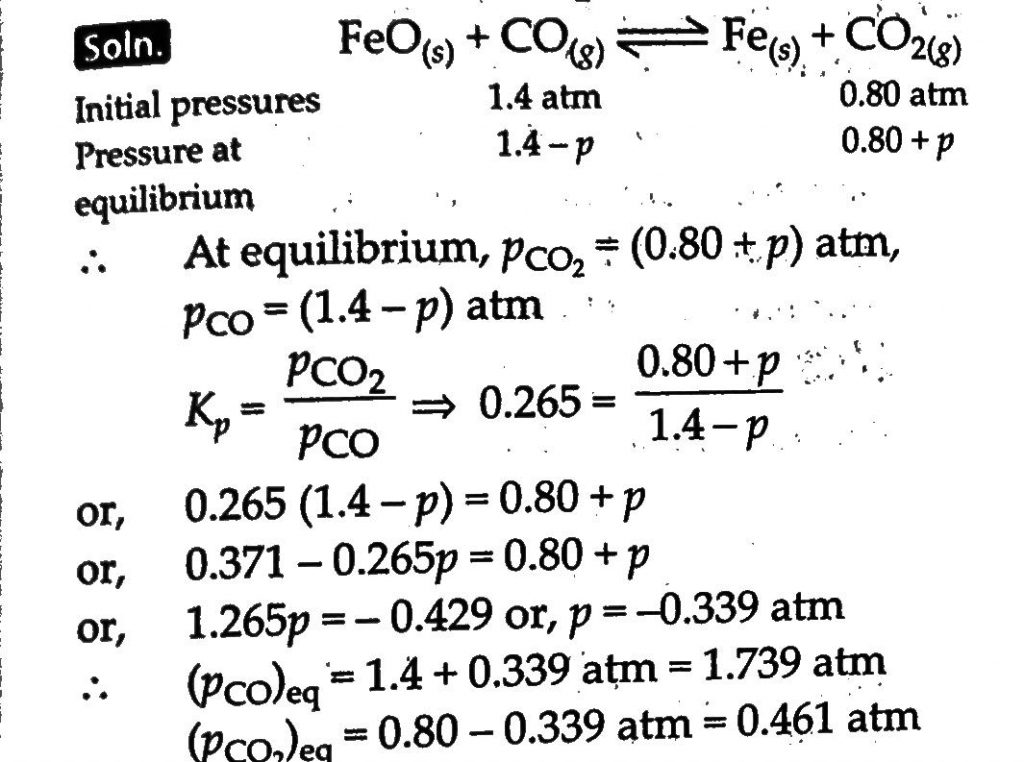
One of the reaction that takes place in producing steel from iron ore is the reduction of iron(II) oxide by carbon monoxide to give iron metal and CO2. FeO(s)+CO(g)⇔Fe(s)+CO2(g),Kp=0.265 atm at 1050K What are the equilibrium partial pressure of CO and CO2 at 1050K if the partical pressure are: pCO=1.4atm and pCO2=0.80atm?