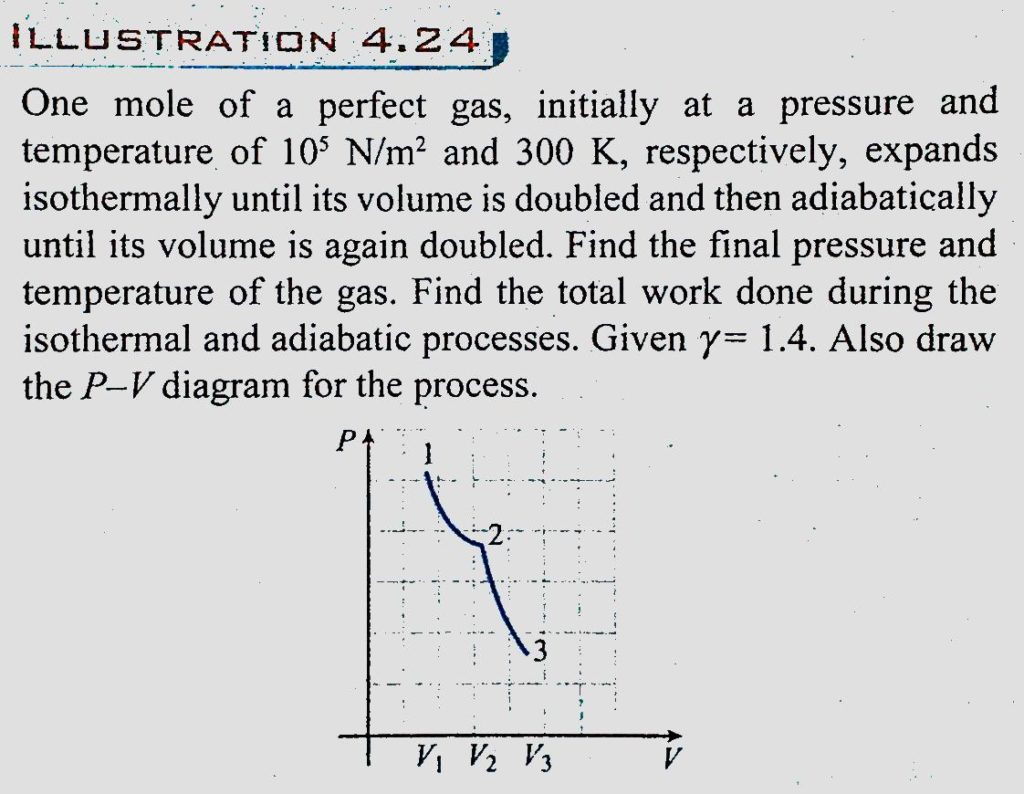
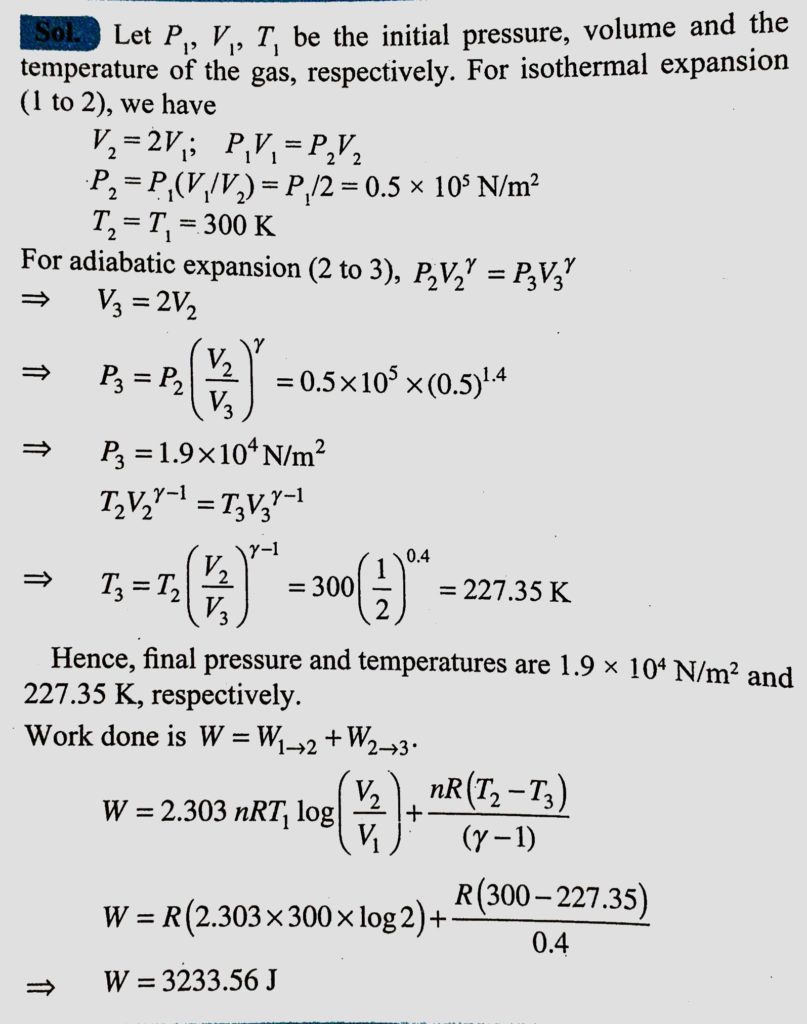
One mole of perfect gas initially at a pressure gas, initially at a pressure and temperature of 10^5 N/m^2 and 300 K, respectively, expands isothermally until its volume is double and then adiabatically until its volume is again doubled. Find the final pressure