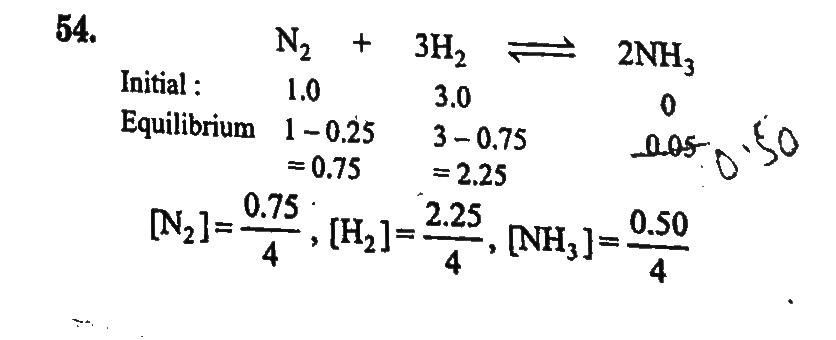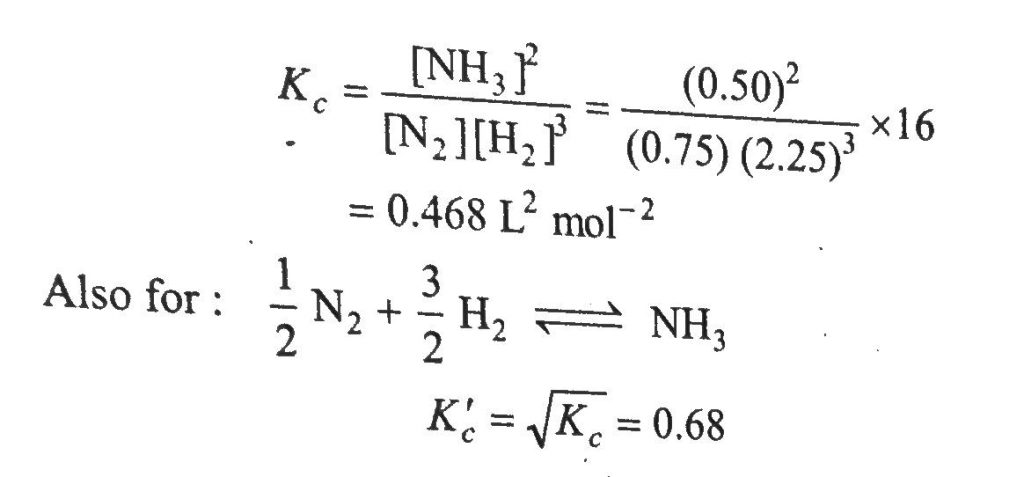One mole of nitrogen is mixed with three moles of hydrogen in a four litre container. If 0.25 per cent of nitrogen is converted to ammonia by the following reaction N2(g)+3H2(g)⇔2NH3(g), then calculate the equilibrium constant, Kc in concentration, units. What will be the value of Kc for the following equilibrium? 1/2N2(g)+3/2H2(g)⇔NH3(g)