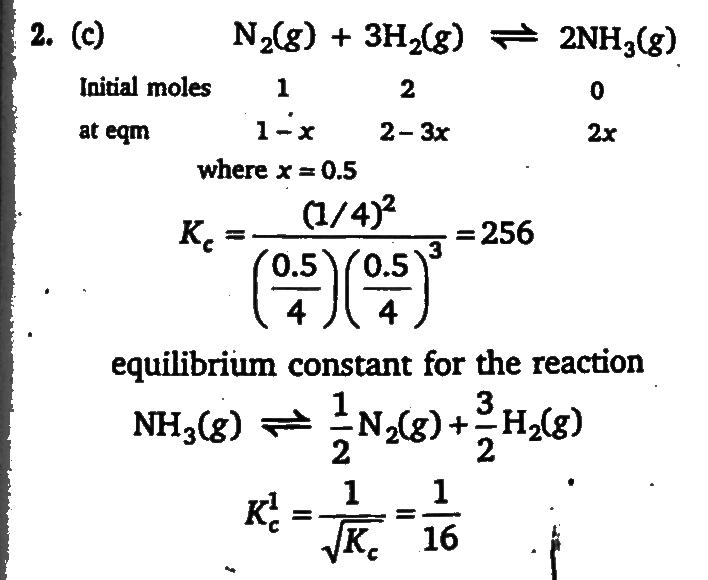One mole of N2 (g) is mixed with 2 moles of H 2(g) in a 4 litre vessel. If 50% of N2(g) is converted to H2(g) by the following reaction : N2 (g)+3H2(g)⇌2NH3 (g) What will be the value of Kc for the following equilibrium? NH3 (g)⇌ 2/1 N2 (g) + 2/3 H2 (g)