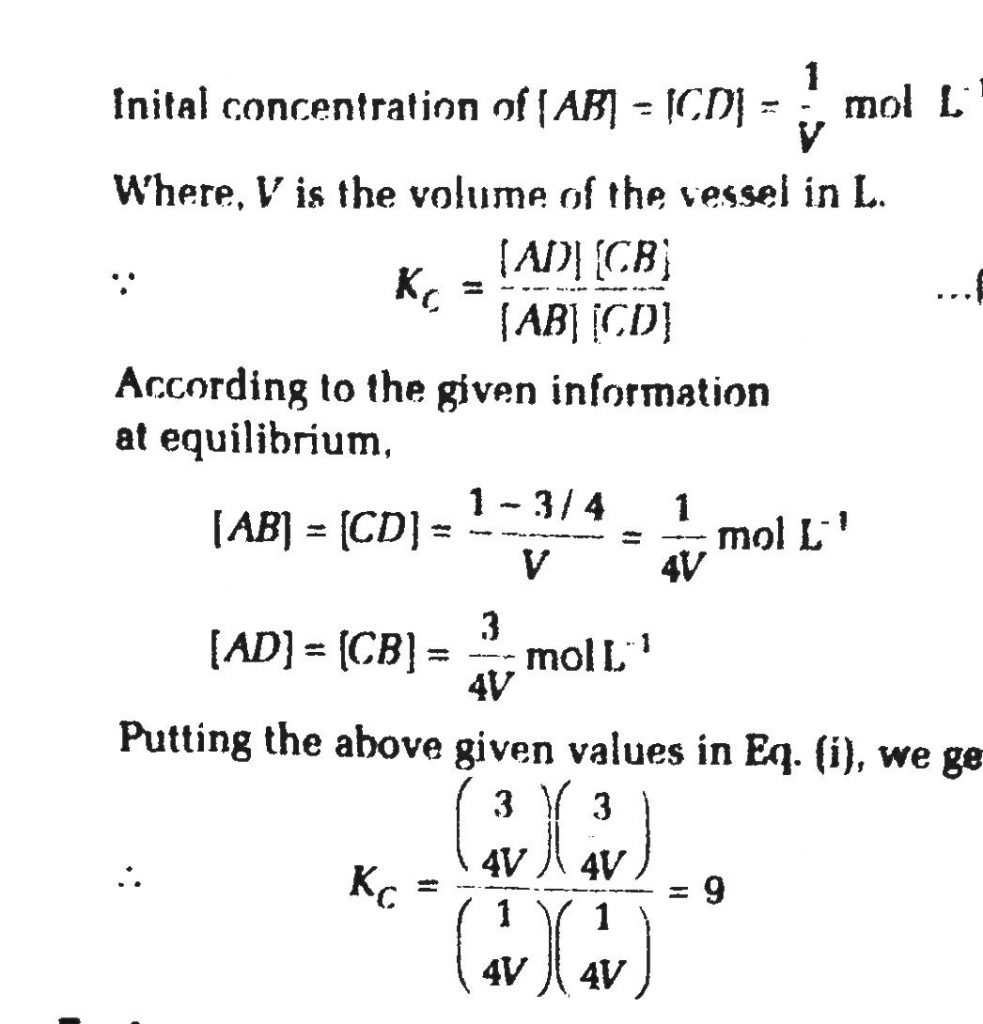
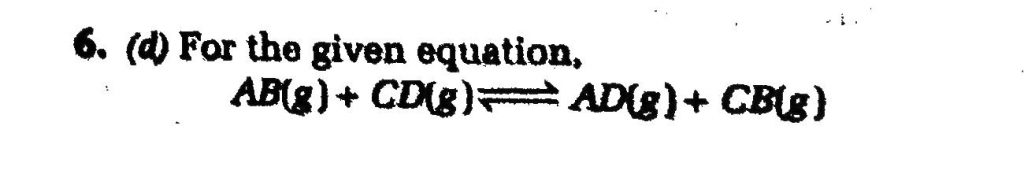One mole of a compound AB reacts with 1 mole of a compound CD according to the equation When equilibrium had been established it was found that 3/4 mole each of reactant AB and CD has been converted to AD and CB. There is no change in volume. The equilibrium constant for the reaction is