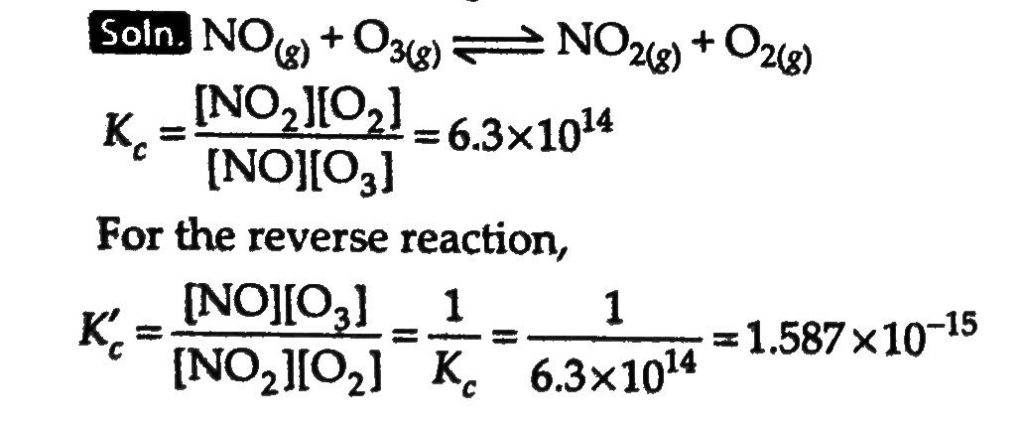Nitric oxide reacts with bromine and gives nitrosyl-bromide as per reaction given below: 2NO(g)+Br2(g)⇔2NOBr(g). When 0.087mole of NO and 0.0437mole of Br2 are mixed in a closed container at constant temperature, 0.0518mole of NOBr is obtained at equilibrium. Calculate equilibrium amount of nitric oxide and bromine.