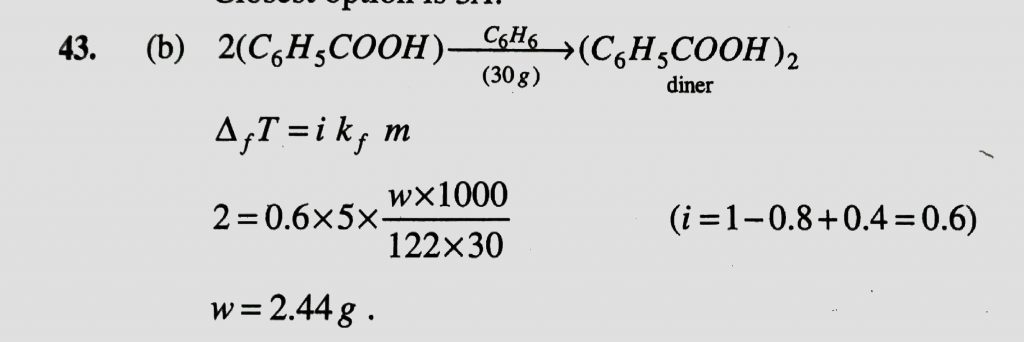Molecules of benzoic acid, (C6H5COOH) dimerise in benzene. ‘w’ g of the acid dissolved in 30 g of benzene point equal to 2K. If the percentage association of the acid to form dimer in the solution is 80, then w is : (Given that Kf = 5 Kg/mol Molar mass of benzoic acid = 122 g/mol )