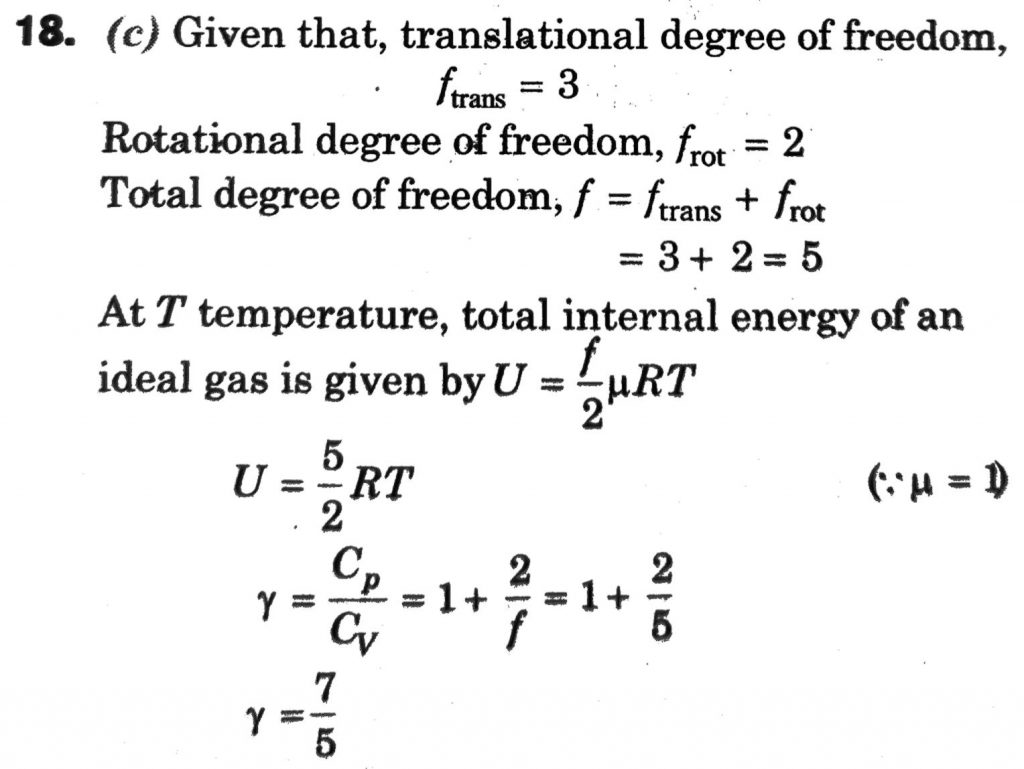Molecules of an ideal gas are known to have three translational degrees of freedom and two rotational degrees of freedom. The gas is maintained at a temperature of T. The total internal energy, U of a mole of this gas, and the value of γ=( Cp/Cv) are given, respectively, by