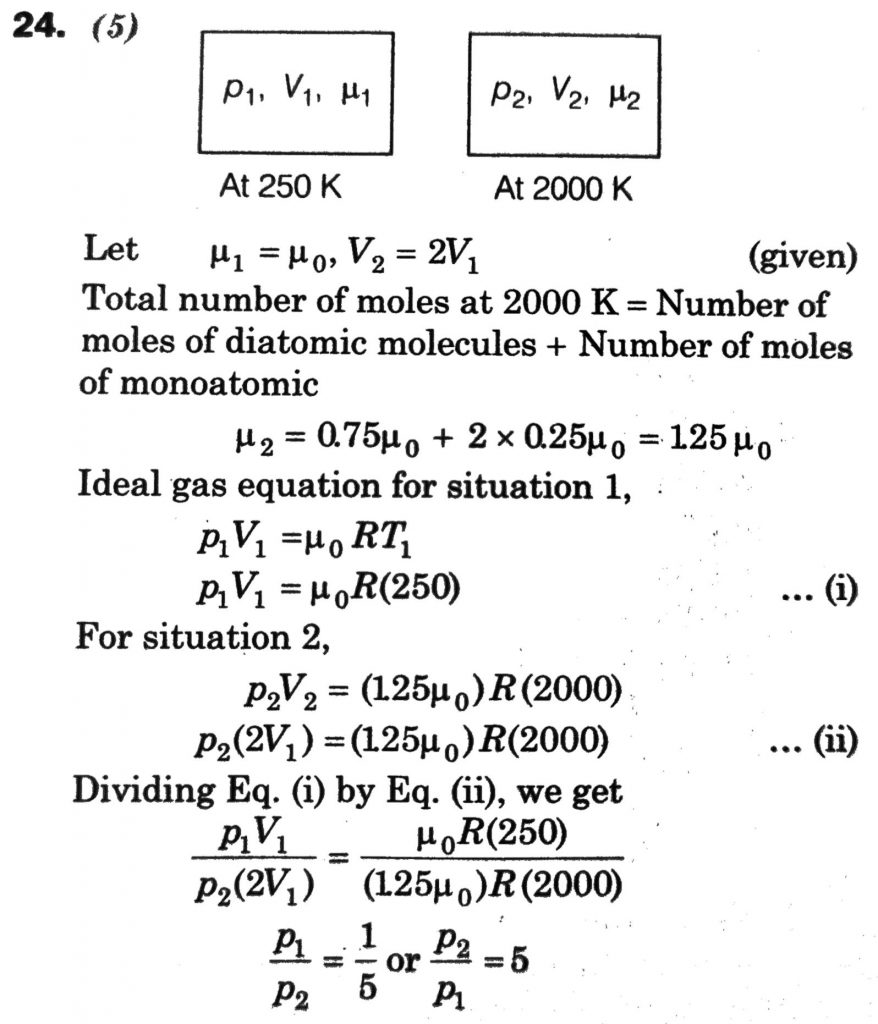
Initially a gas of diatomic molecules is contained in a cylinder of volume V1 at a pressure P1 and temperature 250 K. Assuming that 25% of the molecules get dissociated causing a change in number of moles. The pressure of the resulting gas at temperature 2000 K, when contained in a volume 2V1 is given by P2. The ratio P2/P1 is _________.