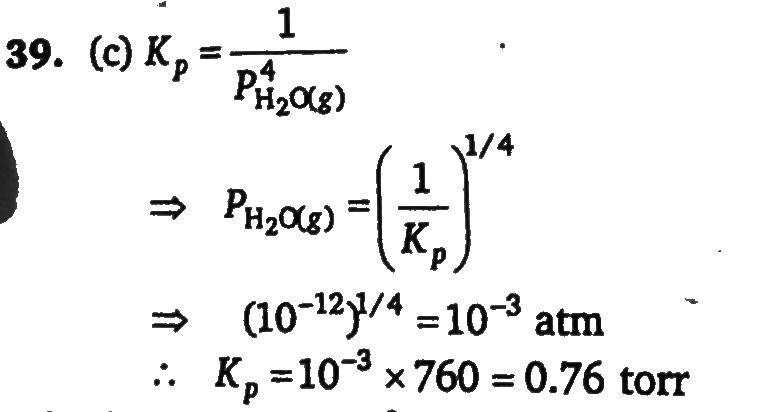In the presence of excess of anhydrous SrCI2 , the amount of water taken up is governed by Kp = 10^12 atm^−4 for the following reaction at 273 K SrCI2 . 2H2O(s) + 4H2O(g) ⇋ SrCl2.6H2O(s) what is equilibrium vapour pressure (in torr) of water in a closed vessel that contains SrCl.2H2 O(s)?