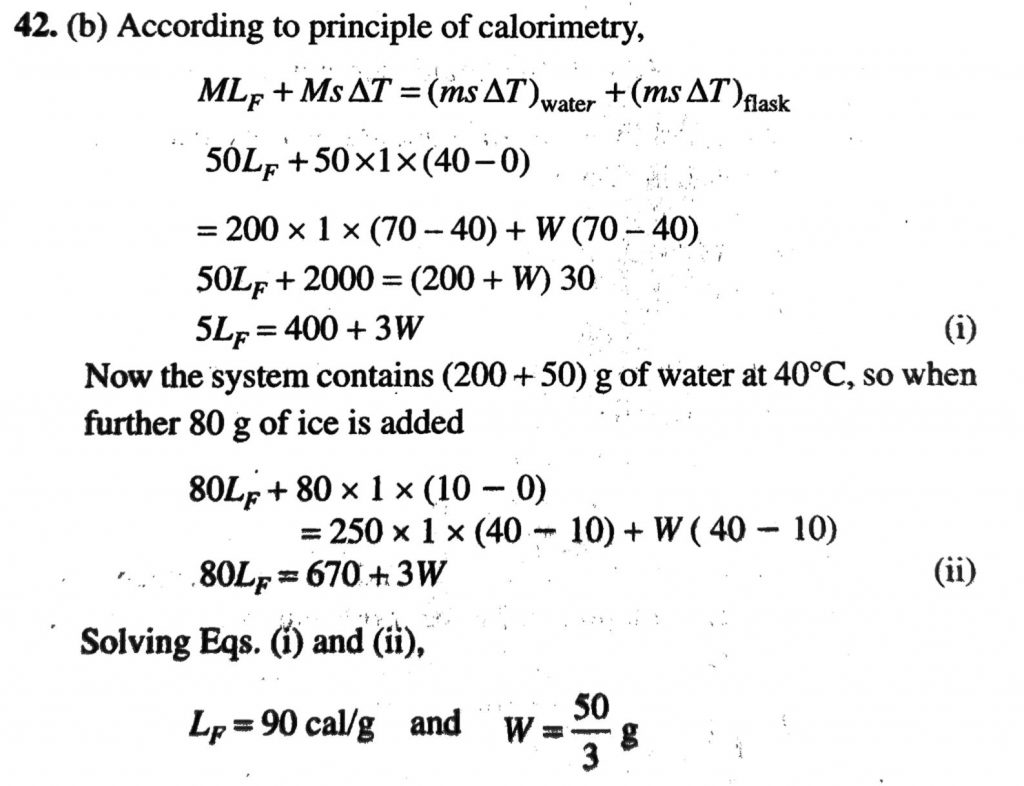
Ice at 0C is added to 200g of water initially at 70C in a vacuum flask. When 50 g of ice has been added and has all melted the temperature of the flask and contents is 40C . When a further 80 g of ice has been added and has all melted, the temperature of the whole is 10C . Neglecting heat to surrounding the latent heat of fusion of ice.