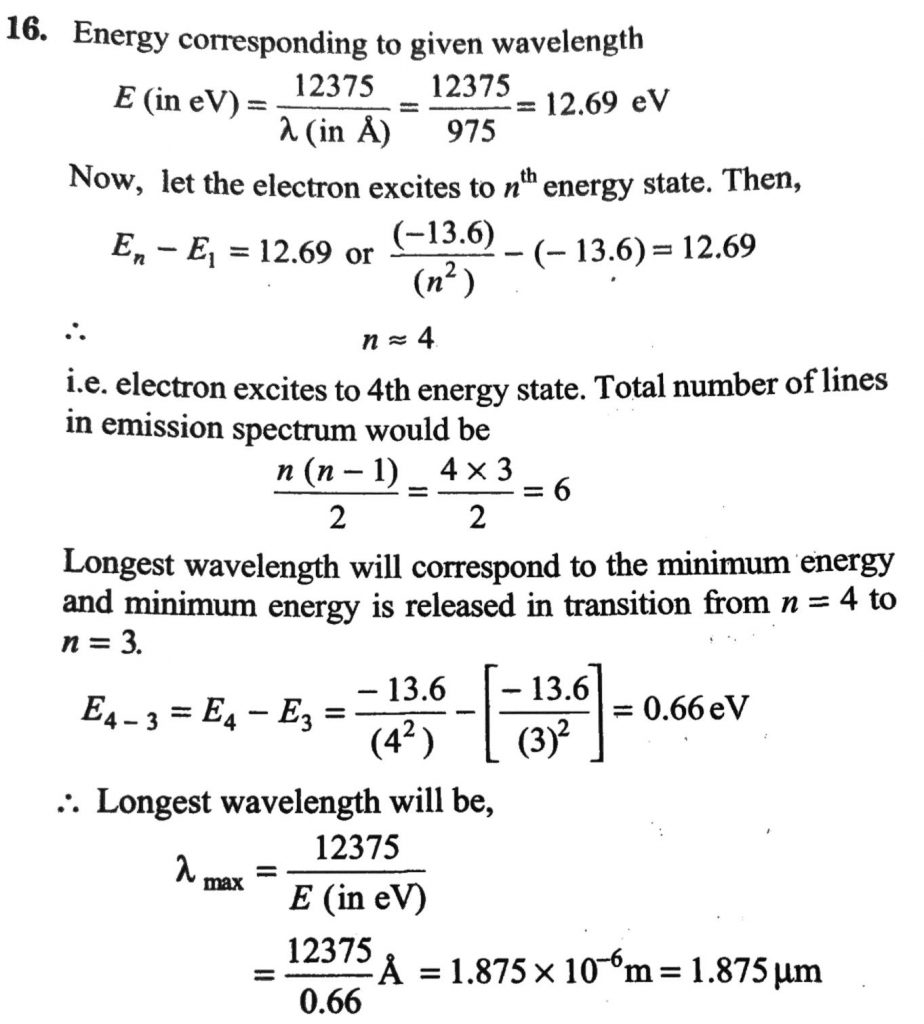
Hydrogen atom in its ground state is excited by means of monochromatic radiation of wavelength 975 A ∘. How many different lines are possible in the resulting spectrum? Calculate the longest wavelength amongst them. You may assume the ionization energy for hydrogen atom as 13.6 eV.