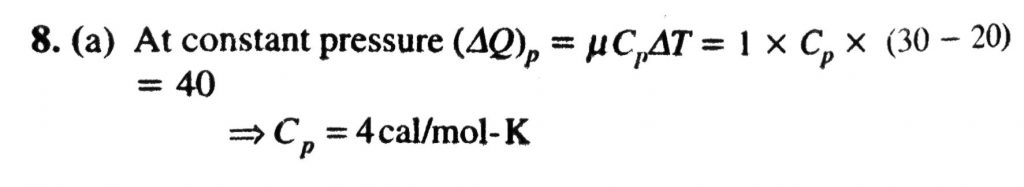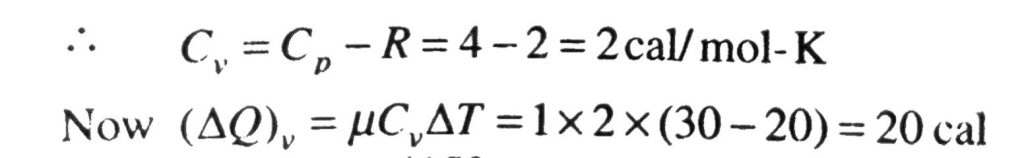Forty calories of heat is needed to raise the temperature of 1 mol of an ideal monatomic gas from 20 C to 30 C at a constant pressure. The amount of heat required to raise its temperature over the same interval at a constant volume (R = 2 cal mol^(-1) K^(-1)) is