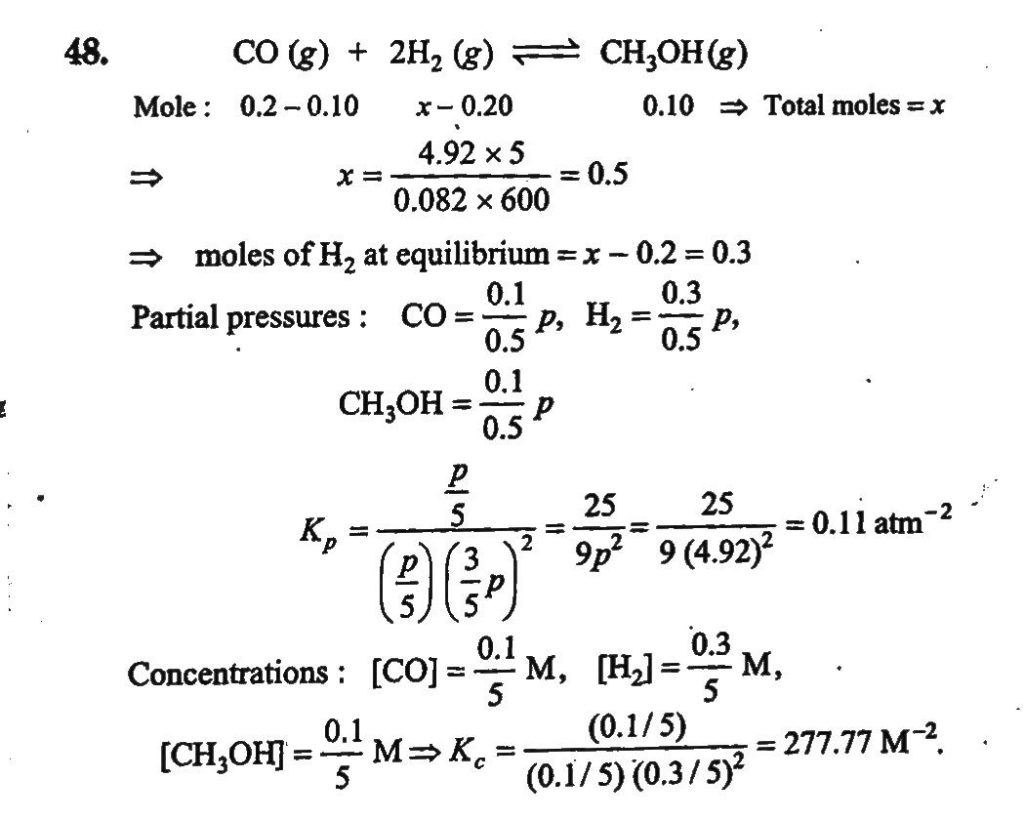
For the reaction, CO(g) + 2H2(g) ⇋ CH3OH(g) hydrogen gas is introduce into a five litre flask at 327° C, containing 0.2 mole of CO(g) and a catalyst, until the pressure is 4.92 atm. At this point 0.1 mole of CH3OH(g) is formed. Calculate the equilibrium constant, Kp and Kc.