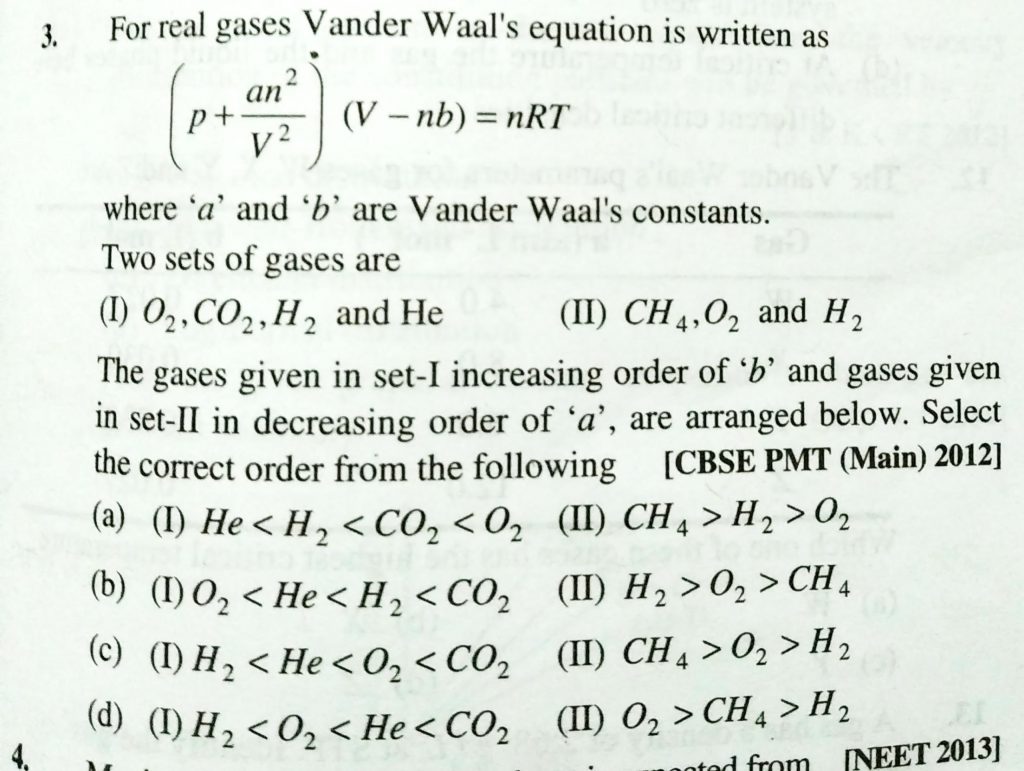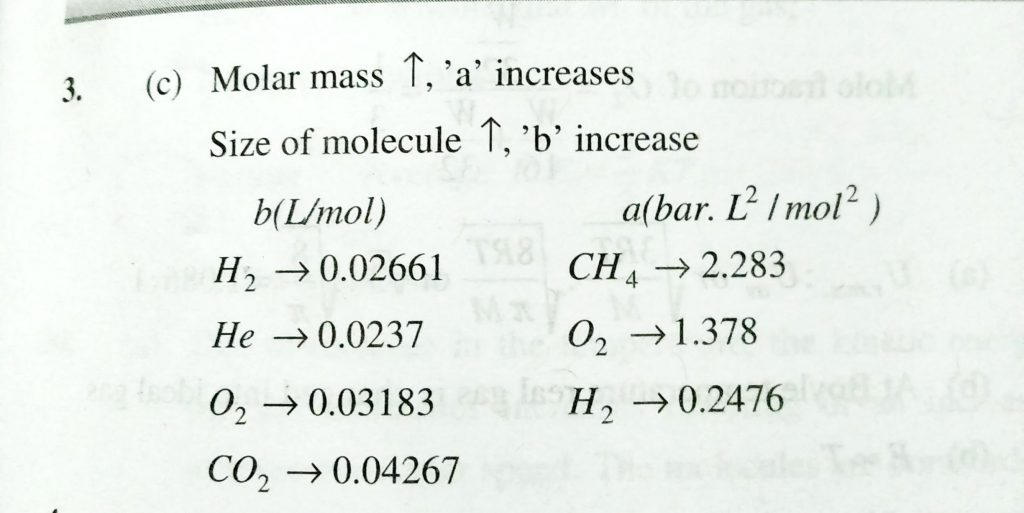For real gases, vander Waals’ equation is written as (P+an^2/V^2) (V − nb) = nRT where a and b are vander Waals’ constants. Two sets of gases are: (I)O2,CO2,H2 and He(II)CH4,O2 and O2andH2 The gases given in set I in increasing order of b and gases given in set II in decreasing order of a are arranged below. Select the correct order from the following: