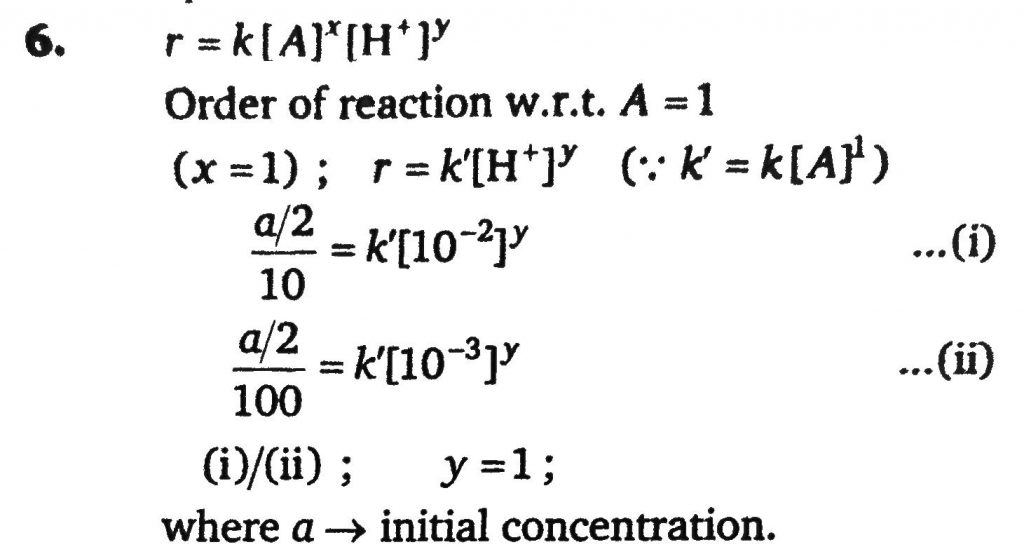For any acid catalysed reaction, AH+→B. half- life period is independent of concentration of A at given pH. At definite concentration of A half- time is 10min at pH = 2 and half- time is 100 min at pH = 3. If the rate law expression of reaction is r = k[A]^x[H+]^y then calculate the value of (x + y).