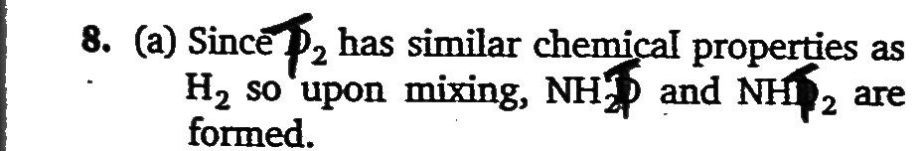For a reversible gaseous reaction N2+3H2 ⇌ 2NH3 at equilibrium ,if some moles of H2 are replaced by same number of moles of T2 (T is tritium, isotope of H and assume isotopes do not have different chemical properties) without affecting other parameters, then: