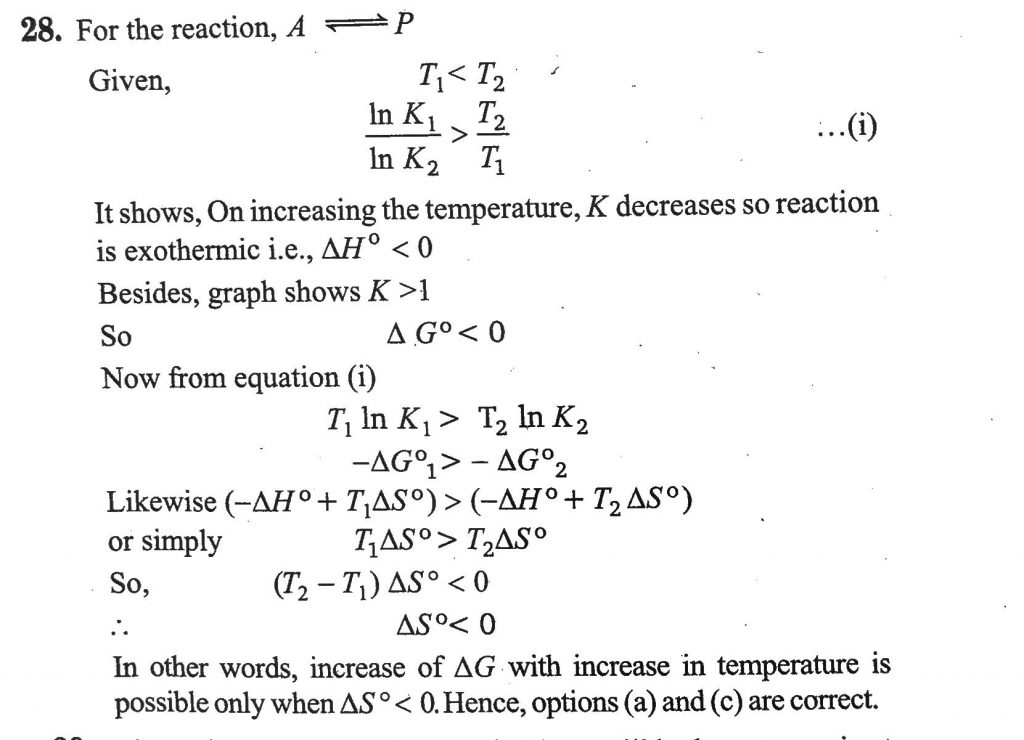
For a reaction, A ⇋ P, the plots of [A] and [P] with time at temperatures T1 and T2 are given below. (Assume ΔHθ and ΔSθ are independent of temperature and ratio of ln K at T1 to ln K at T2 is greater than T2/T1 . Here H, S, G and K are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively.)