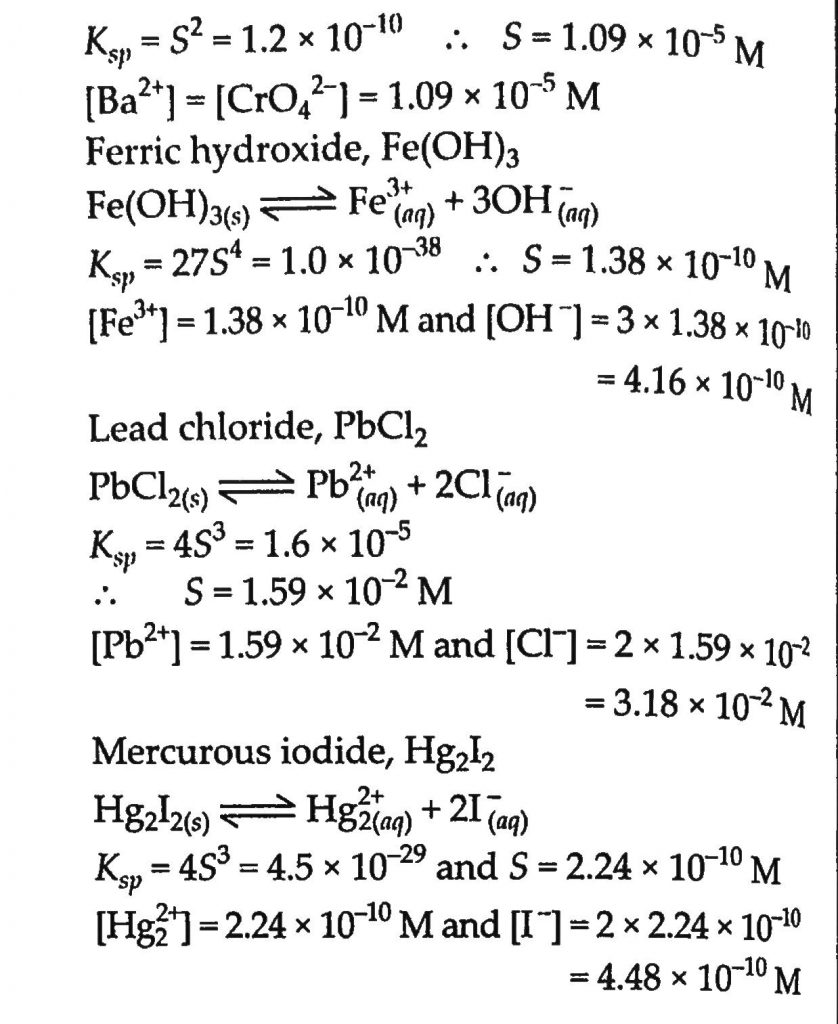
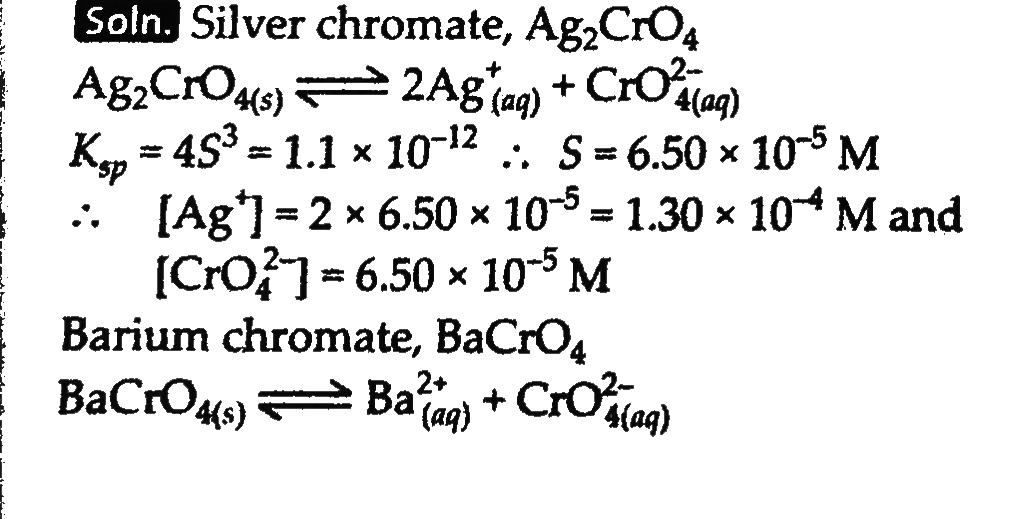Determine the solubilities of silver chromate, barium chromate, ferric hydroxide, lead chloride and mercurous iodide at 298 K from their solubility product constants. Determine also the molarities of individual ions. The solubility product constants are: Silver chromate, 1.1×10 −12Barium chromate, 1.2×10 −10Ferric hydroxide, 1.0×10 −38Lead dichloride, 1.6×10 −5Mercurous iodide, 4.5×10 −29