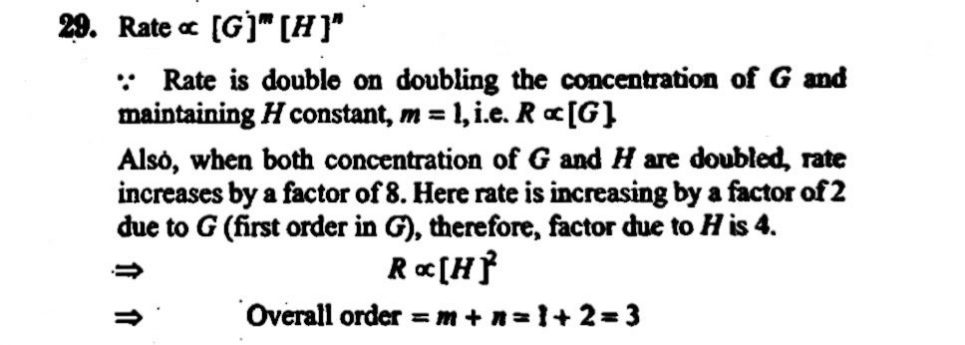Consider the reaction aG + bH→ Products When the concentration of both the reactants G and H is doubled, the rate increases by eight times. However, when the concentration of G is doubled keeping the concentration of H fixed, the rate is doubled. The over all order of reaction is