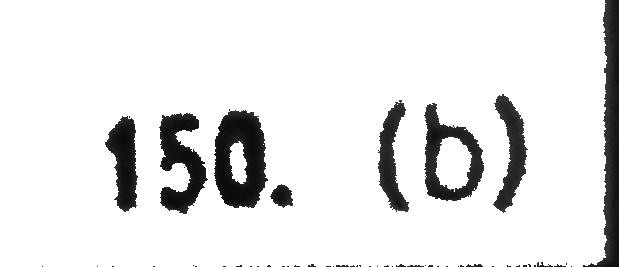Consider the following statement and select correct option: (I) Ksp of Fe(OH)3 in aqueous solution is 3.8×10^−38 at 298 K. The concentration of Fe+ will increase when [H+] ion concentration decreases. (II) In a mixture of NH4Cl and NH4OH in water, a further amount of NH4Cl is added. The pH of the mixture will decreases. (III) An aqueous solution of each of the following salt (NH4I,HCOOK) will be basic, acidic respectively.