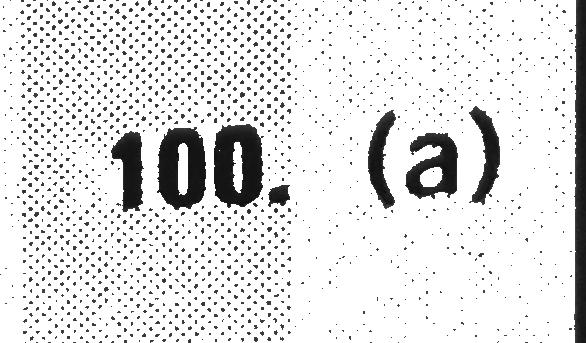Consider the following reaction at equilibrium and determine which of the indicated changes will cause the reaction to proceed to right. (1)CO(g)+3H2(g)⇔CH4(g)+H2O(g) (add CH4) (2)N2(g)+3H2(g)⇔2NH3(g) (removeNH3) (3) H_(2)(g)+F_(2)(g)⇔2HF(g) (add F_(2))(4)BaO(s)+SO_(3)(g)⇔BASO_(4)(s) (add BaO)`