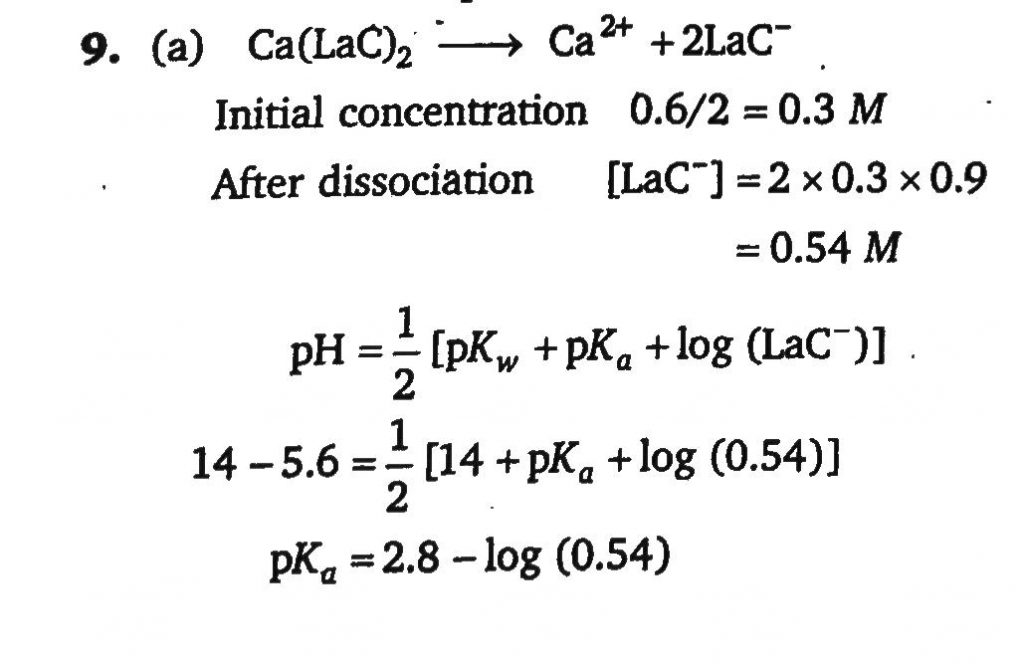
Calcium lactate is a salt of weal organic acid and strong base represented as Ca(LaC)2. A saturated solution of Ca(LaC)2 contains 0.6 mole in 2 litre solution. pOH of solution is 5.60. If 90% dissociation of the salt takes place then what is pKa of lactic acid?