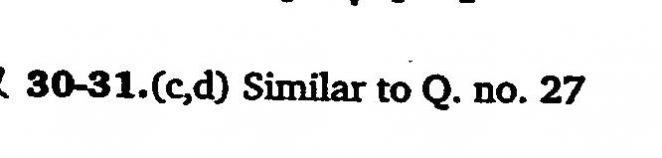At a certain temperature, the following reactions have the equilibrium constants as shown below S(s)+O2(g) ⇌ SO2(g);Kc1 = 5×10^52 2S(s) + 3O2 (g) ⇌ 2SO3 (g); Kc2 =10^29 What is the equilibrium constant, Kc for the reaction at the same temperature ? 2SO2 (g)+O2(g) ⇌ 2SO3 (g)