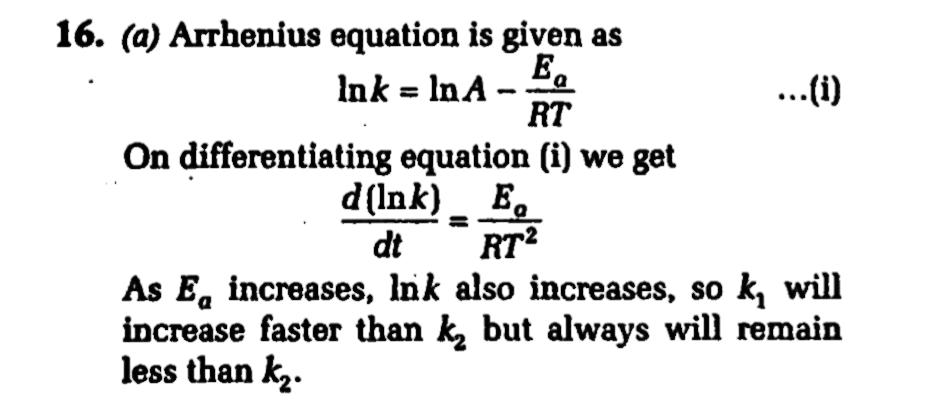At a certain temperature, the first order rate constant, k1 is found to be smaller than the second order rate constant, k2 . If the energy of activation, E1 of the first order reaction is greater than energy of activation, E2 of the second order reaction then with increase in temperature.