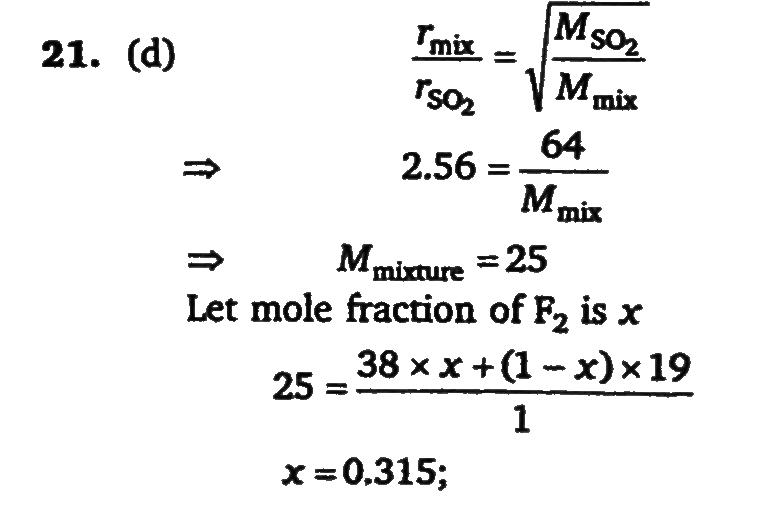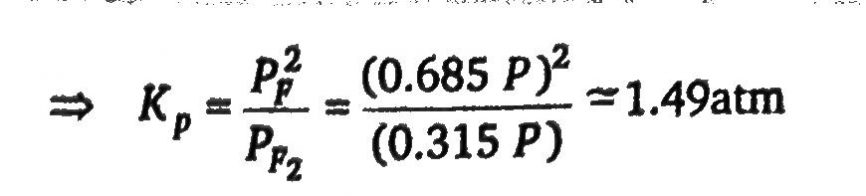At 800 degree C, the following equilibrium is established as: F2(g)⇌2F(g) The composition of equilibrium may be determined by measuring the rate of effusion of the mixture through a pinhole. It is found that at 800^oC and 1 atm mixture effuses 1.6 times as fast as SO2 effuses under the similar conditions. (At.wt. of F=19). What is the value of Kp (in atm)?