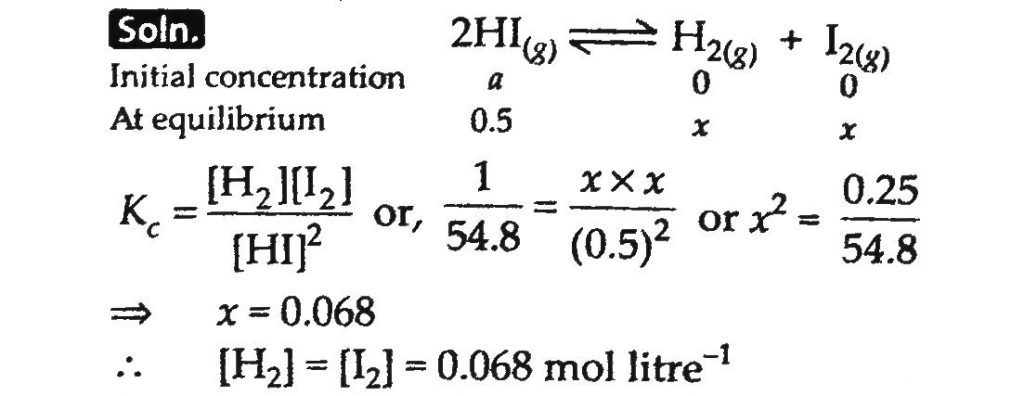At 700 K equilibrium constant for the reaction; H2(g) + I2(g) ⇌ 2HI(g) is 54.8. If 0.5 mol litre^ −1 of HI(g) is present at equilibrium at 700 K, what are the concentrations of H2(g) and I2(g) assuming that we initially started with HI(g) and allowed it to reach equilibrium at 700 K.