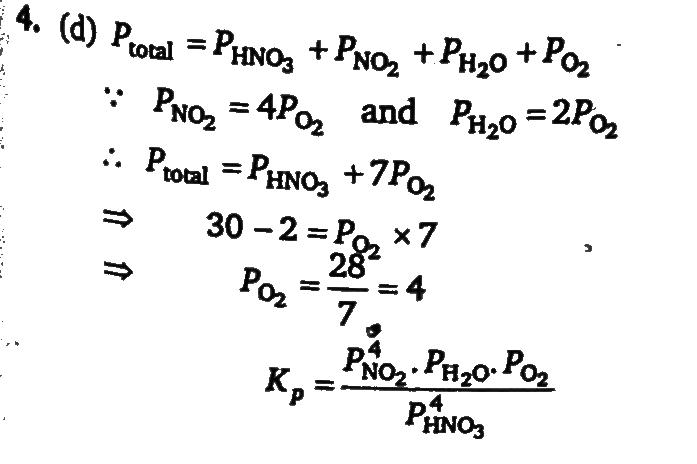
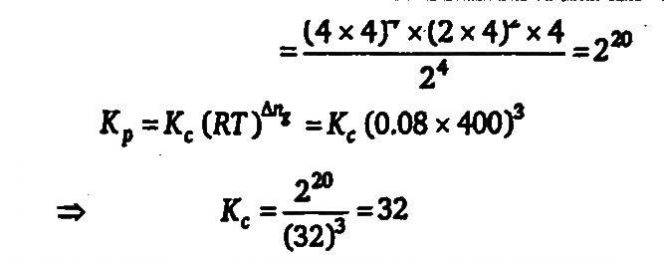Assume that the decomposition of HNO3 can be represented by the following equation 4HNO3(g)⇔4NO2(g)+2H2O(g)+O2(g) and the reaction approaches equilibrium at 400K temperature and 30 atm pressure. At equilibrium partial pressure of HNO3 is 2 atm Calculate Kc in (mol/L−K) at 400K (Use:R=0.08atm−L/mol−K)